Cardiac-Specific Deletion of Pyruvate Dehydrogenase Impairs Glucose Oxidation Rates and Induces Diastolic Dysfunction
- PMID: 29560354
- PMCID: PMC5845646
- DOI: 10.3389/fcvm.2018.00017
Cardiac-Specific Deletion of Pyruvate Dehydrogenase Impairs Glucose Oxidation Rates and Induces Diastolic Dysfunction
Abstract
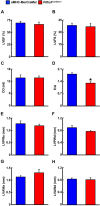
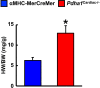
Obesity and type 2 diabetes (T2D) increase the risk for cardiomyopathy, which is the presence of ventricular dysfunction in the absence of underlying coronary artery disease and/or hypertension. As myocardial energy metabolism is altered during obesity/T2D (increased fatty acid oxidation and decreased glucose oxidation), we hypothesized that restricting myocardial glucose oxidation in lean mice devoid of the perturbed metabolic milieu observed in obesity/T2D would produce a cardiomyopathy phenotype, characterized via diastolic dysfunction. We tested our hypothesis via producing mice with a cardiac-specific gene knockout for pyruvate dehydrogenase (PDH, gene name Pdha1), the rate-limiting enzyme for glucose oxidation. Cardiac-specific Pdha1 deficient (Pdha1Cardiac-/-) mice were generated via crossing a tamoxifen-inducible Cre expressing mouse under the control of the alpha-myosin heavy chain (αMHC-MerCreMer) promoter with a floxed Pdha1 mouse. Energy metabolism and cardiac function were assessed via isolated working heart perfusions and ultrasound echocardiography, respectively. Tamoxifen administration produced an ~85% reduction in PDH protein expression in Pdha1Cardiac-/- mice versus their control littermates, which resulted in a marked reduction in myocardial glucose oxidation and a corresponding increase in palmitate oxidation. This myocardial metabolism profile did not impair systolic function in Pdha1Cardiac-/- mice, which had comparable left ventricular ejection fractions and fractional shortenings as their αMHC-MerCreMer control littermates, but did produce diastolic dysfunction as seen via the reduced mitral E/A ratio. Therefore, it does appear that forced restriction of glucose oxidation in the hearts of Pdha1Cardiac-/- mice is sufficient to produce a cardiomyopathy-like phenotype, independent of the perturbed metabolic milieu observed in obesity and/or T2D.
Keywords: cardiac function; diabetic cardiomyopathy; diastolic dysfunction; glucose oxidation; pyruvate dehydrogenase.
Figures




Similar articles
-
Barth syndrome-related cardiomyopathy is associated with a reduction in myocardial glucose oxidation.Am J Physiol Heart Circ Physiol. 2021 Jun 1;320(6):H2255-H2269. doi: 10.1152/ajpheart.00873.2020. Epub 2021 Apr 30. Am J Physiol Heart Circ Physiol. 2021. PMID: 33929899
-
Liraglutide alleviates experimental diabetic cardiomyopathy in a PDH-dependent manner.J Endocrinol. 2024 Jul 8;262(2):e240032. doi: 10.1530/JOE-24-0032. Print 2024 Aug 1. J Endocrinol. 2024. PMID: 38860519
-
The GLP-1 Receptor Agonist Liraglutide Increases Myocardial Glucose Oxidation Rates via Indirect Mechanisms and Mitigates Experimental Diabetic Cardiomyopathy.Can J Cardiol. 2021 Jan;37(1):140-150. doi: 10.1016/j.cjca.2020.02.098. Epub 2020 Mar 8. Can J Cardiol. 2021. PMID: 32640211
-
Inhibition of Pyruvate Dehydrogenase in the Heart as an Initiating Event in the Development of Diabetic Cardiomyopathy.Antioxidants (Basel). 2023 Mar 20;12(3):756. doi: 10.3390/antiox12030756. Antioxidants (Basel). 2023. PMID: 36979003 Free PMC article. Review.
-
Pyruvate Dehydrogenase Complex and Glucose Oxidation as a Therapeutic Target in Diabetic Heart Disease.J Lipid Atheroscler. 2023 Jan;12(1):47-57. doi: 10.12997/jla.2023.12.1.47. Epub 2022 Nov 7. J Lipid Atheroscler. 2023. PMID: 36761067 Free PMC article. Review.
Cited by
-
Biochemical Aspects That Lead to Abusive Use of Trimetazidine in Performance Athletes: A Mini-Review.Int J Mol Sci. 2024 Jan 28;25(3):1605. doi: 10.3390/ijms25031605. Int J Mol Sci. 2024. PMID: 38338885 Free PMC article. Review.
-
Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency.Cardiovasc Res. 2019 Sep 1;115(11):1606-1616. doi: 10.1093/cvr/cvz045. Cardiovasc Res. 2019. PMID: 30778524 Free PMC article.
-
Conditional knockout of pyruvate dehydrogenase in mouse pancreatic β‑cells causes morphological and functional changes.Mol Med Rep. 2020 Apr;21(4):1717-1726. doi: 10.3892/mmr.2020.10993. Epub 2020 Feb 20. Mol Med Rep. 2020. PMID: 32319629 Free PMC article.
-
Amyloid beta 42 alters cardiac metabolism and impairs cardiac function in male mice with obesity.Nat Commun. 2024 Jan 15;15(1):258. doi: 10.1038/s41467-023-44520-4. Nat Commun. 2024. PMID: 38225272 Free PMC article.
-
PPARγ Agonist Pioglitazone Prevents Hypoxia-induced Cardiac Dysfunction by Reprogramming Glucose Metabolism.Int J Biol Sci. 2024 Aug 6;20(11):4297-4313. doi: 10.7150/ijbs.98387. eCollection 2024. Int J Biol Sci. 2024. PMID: 39247816 Free PMC article.
References
-
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology (2005) 146(12):5341–9.10.1210/en.2005-0938 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

