CD4 T cells react to local increase of α-synuclein in a pathology-associated variant-dependent manner and modify brain microglia in absence of brain pathology
- PMID: 29560431
- PMCID: PMC5857520
- DOI: 10.1016/j.heliyon.2018.e00513
CD4 T cells react to local increase of α-synuclein in a pathology-associated variant-dependent manner and modify brain microglia in absence of brain pathology
Abstract
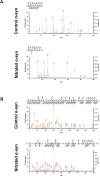
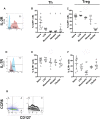
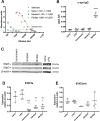
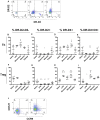
We have previously shown that immunological processes in the brain during α-synuclein-induced neurodegeneration vary depending on the presence or absence of cell death. This suggests that the immune system is able to react differently to the different stages of α-synuclein pathology. However, it was unclear whether these immune changes were governed by brain processes or by a direct immune response to α-synuclein modifications. We have herein locally increased the peripheral concentration of α-synuclein or its pathology-associated variants, nitrated or fibrillar, to characterize the modulation of the CD4 T cell pool by α-synuclein and brain microglia in the absence of any α-synuclein brain pathology. We observed that α-synuclein changed the CD4:CD8 ratio by contracting the CD3+CD4+ T cell pool and reducing the pool of memory Regulatory T cells (Treg). Nitrated α-synuclein induced the expansion of both the CD3+CD4+ and CD3+CD4- T cells, while fibrils increased the percentage of Foxp3+ Treg cells and induced anti-α-synuclein antibodies. Furthermore, the activation pattern of CD3+CD4+ T cells was modulated in a variant-dependent manner; while nitrated and fibrillar α-synuclein expanded the fraction of activated Treg, all three α-synuclein variants reduced the expression levels of STAT3, CD25 and CD127 on CD3+CD4+ T cells. Additionally, while monomeric α-synuclein increased CD103 expression, the fibrils decreased it, and CCR6 expression was decreased by nitrated and fibrillar α-synuclein, indicating that α-synuclein variants affect the homing and tolerance capacities of CD3+CD4+ T cells. Indeed, this correlated with changes in brain microglia phenotype, as determined by FACS analysis, in an α-synuclein variant-specific manner and coincided in time with CD4+ T cell infiltration into brain parenchyma. We have shown that the peripheral immune system is able to sense and react specifically to changes in the local concentration and structure of α-synuclein, which results in variant-specific T cell migration into the brain. This may have a specific repercussion for brain microglia.
Keywords: Immunology; Neuroscience.
Figures





Similar articles
-
α-Synuclein vaccination modulates regulatory T cell activation and microglia in the absence of brain pathology.J Neuroinflammation. 2016 Apr 7;13(1):74. doi: 10.1186/s12974-016-0532-8. J Neuroinflammation. 2016. PMID: 27055651 Free PMC article.
-
[The subpopulation CD4(+); CD25(+); Foxp3(+);/CD127(low/-); regulatory T cells in peripheral blood of HIV-infected patients correlated with disease progression].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012 Nov;28(11):1188-91. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012. PMID: 23127412 Chinese.
-
Differences in the induction of induced human CD4(+) CD25(+) FoxP3(+) T-regulatory cells and CD3(+) CD8(+) CD28(-) T-suppressor cells subset phenotypes in vitro: comparison of phorbol 12-myristate 13-acetate/ionomycin and phytohemagglutinin stimulation.Transplant Proc. 2013 Jun;45(5):1822-31. doi: 10.1016/j.transproceed.2012.10.061. Transplant Proc. 2013. PMID: 23769052
-
Foxp3(high) and Foxp3(low) Treg cells differentially correlate with T helper 1 and natural killer cells in peripheral blood.Hum Immunol. 2011 Aug;72(8):621-6. doi: 10.1016/j.humimm.2011.03.013. Epub 2011 May 11. Hum Immunol. 2011. PMID: 21600259
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
Cited by
-
Microglia Response During Parkinson's Disease: Alpha-Synuclein Intervention.Front Cell Neurosci. 2018 Aug 6;12:247. doi: 10.3389/fncel.2018.00247. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30127724 Free PMC article. Review.
-
Periphery and brain, innate and adaptive immunity in Parkinson's disease.Acta Neuropathol. 2021 Apr;141(4):527-545. doi: 10.1007/s00401-021-02268-5. Epub 2021 Feb 8. Acta Neuropathol. 2021. PMID: 33555429 Free PMC article. Review.
-
Neuromodulation influences T lymphocyte calcium signaling and alpha synuclein clearance: implications for Parkinson's disease.Front Cell Neurosci. 2025 Jul 18;19:1627305. doi: 10.3389/fncel.2025.1627305. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40756016 Free PMC article. Review.
-
The Interplay between α-Synuclein and Microglia in α-Synucleinopathies.Int J Mol Sci. 2023 Jan 27;24(3):2477. doi: 10.3390/ijms24032477. Int J Mol Sci. 2023. PMID: 36768798 Free PMC article. Review.
-
Alpha synuclein in hematopoiesis and immunity.Heliyon. 2019 Oct 18;5(10):e02590. doi: 10.1016/j.heliyon.2019.e02590. eCollection 2019 Oct. Heliyon. 2019. PMID: 31692680 Free PMC article. Review.
References
-
- Almeida A.R., Legrand N., Papiernik M., Freitas A.A. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J. Immunol. 2002;169(9):4850–4860. - PubMed
-
- Almeida A.R., Zaragoza B., Freitas A.A. Indexation as a novel mechanism of lymphocyte homeostasis: the number of CD4+CD25+ regulatory T cells is indexed to the number of IL-2-producing cells. J. Immunol. 2006;177(1):192–200. - PubMed
-
- Baba Y., Kuroiwa A., Uitti R.J., Wszolek Z.K., Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat. Disord. 2005;11(8):493–498. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

