Biochemical alterations in inflammatory reactive chondrocytes: evidence for intercellular network communication
- PMID: 29560438
- PMCID: PMC5857518
- DOI: 10.1016/j.heliyon.2018.e00525
Biochemical alterations in inflammatory reactive chondrocytes: evidence for intercellular network communication
Abstract
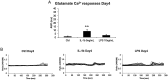
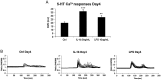
Chondrocytes are effectively involved in the pathophysiological processes of inflammation in joints. They form cellular processes in the superficial layer of the articular cartilage and form gap junction coupled syncytium to facilitate cell-to-cell communication. However, very little is known about their physiological cellular identity and communication. The aim with the present work is to evaluate the physiological behavior after stimulation with the inflammatory inducers interleukin-1β and lipopolysaccharide. The cytoskeleton integrity and intracellular Ca2+ release were assessed as indicators of inflammatory state. Cytoskeleton integrity was analyzed through cartilage oligomeric matrix protein and actin labeling with an Alexa 488-conjugated phalloidin probe. Ca2+ responses were assessed through the Ca2+ sensitive fluorophore Fura-2/AM. Western blot analyses of several inflammatory markers were performed. The results show reorganization of the actin filaments. Glutamate, 5-hydoxytryptamine, and ATP evoked intracellular Ca2+ release changed from single peaks to oscillations after inflammatory induction in the chondrocytes. The expression of toll-like receptor 4, the glutamate transporters GLAST and GLT-1, and the matrix metalloproteinase-13 increased. This work demonstrates that chondrocytes are a key part in conditions that lead to inflammation in the cartilage. The inflammatory inducers modulate the cytoskeleton, the Ca2+ signaling, and several inflammatory parameters. In conclusion, our data show that the cellular responses to inflammatory insults from healthy and inflammatory chondrocytes resemble those previously observed in astrocyte and cardiac fibroblasts networks.
Keywords: Cell biology.
Figures





Similar articles
-
Sildenafil (Viagra(®)) prevents and restores LPS-induced inflammation in astrocytes.Neurosci Lett. 2016 Sep 6;630:59-65. doi: 10.1016/j.neulet.2016.07.029. Epub 2016 Jul 25. Neurosci Lett. 2016. PMID: 27466020
-
Anti-inflammatory effects induced by pharmaceutical substances on inflammatory active brain astrocytes-promising treatment of neuroinflammation.J Neuroinflammation. 2018 Nov 17;15(1):321. doi: 10.1186/s12974-018-1361-8. J Neuroinflammation. 2018. PMID: 30447700 Free PMC article.
-
Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of toll-like receptor 4 and antagonized by bone morphogenetic protein 7.Arthritis Rheum. 2007 Jun;56(6):1880-93. doi: 10.1002/art.22637. Arthritis Rheum. 2007. PMID: 17530716
-
Effects of shear stress on articular chondrocyte metabolism.Biorheology. 2000;37(1-2):95-107. Biorheology. 2000. PMID: 10912182 Review.
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
Cited by
-
A randomized, triple-blinded controlled clinical study with a novel disease-modifying drug combination in equine lameness-associated osteoarthritis.Osteoarthr Cartil Open. 2023 Jun 16;5(3):100381. doi: 10.1016/j.ocarto.2023.100381. eCollection 2023 Sep. Osteoarthr Cartil Open. 2023. PMID: 37416846 Free PMC article.
-
3D bioprinted scaffolds for osteochondral regeneration: advancements and applications.Mater Today Bio. 2025 May 8;32:101834. doi: 10.1016/j.mtbio.2025.101834. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40487176 Free PMC article. Review.
-
Ameliorative Effects of Loganin on Arthritis in Chondrocytes and Destabilization of the Medial Meniscus-Induced Animal Model.Pharmaceuticals (Basel). 2021 Feb 8;14(2):135. doi: 10.3390/ph14020135. Pharmaceuticals (Basel). 2021. PMID: 33567513 Free PMC article.
-
Pathologically altered articular cartilage attracts intense chondrocyte invasion into the extracellular matrix: in vitro pilot study.Knee Surg Relat Res. 2024 Dec 3;36(1):42. doi: 10.1186/s43019-024-00249-y. Knee Surg Relat Res. 2024. PMID: 39627845 Free PMC article.
-
Bupivacaine in combination with sildenafil (Viagra) and vitamin D3 have anti-inflammatory effects in osteoarthritic chondrocytes.Curr Res Pharmacol Drug Discov. 2021 Oct 19;2:100066. doi: 10.1016/j.crphar.2021.100066. eCollection 2021. Curr Res Pharmacol Drug Discov. 2021. PMID: 34909684 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

