Chronic voluntary oral methamphetamine induces deficits in spatial learning and hippocampal protein kinase Mzeta with enhanced astrogliosis and cyclooxygenase-2 levels
- PMID: 29560440
- PMCID: PMC5857642
- DOI: 10.1016/j.heliyon.2018.e00509
Chronic voluntary oral methamphetamine induces deficits in spatial learning and hippocampal protein kinase Mzeta with enhanced astrogliosis and cyclooxygenase-2 levels
Abstract
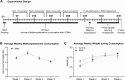
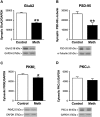
Methamphetamine (MA) is an addictive drug with neurotoxic effects on the brain producing cognitive impairment and increasing the risk for neurodegenerative disease. Research has focused largely on examining the neurochemical and behavioral deficits induced by injecting relatively high doses of MA [30 mg/kg of body weight (bw)] identifying the upper limits of MA-induced neurotoxicity. Accordingly, we have developed an appetitive mouse model of voluntary oral MA administration (VOMA) based on the consumption of a palatable sweetened oatmeal mash containing a known amount of MA. This VOMA model is useful for determining the lower limits necessary to produce neurotoxicity in the short-term and long-term as it progresses over time. We show that mice consumed on average 1.743 mg/kg bw/hour during 3 hours, and an average of 5.23 mg/kg bw/day over 28 consecutive days on a VOMA schedule. Since this consumption rate is much lower than the neurotoxic doses typically injected, we assessed the effects of long-term chronic VOMA on both spatial memory performance and on the levels of neurotoxicity in the hippocampus. Following 28 days of VOMA, mice exhibited a significant deficit in short-term spatial working memory and spatial reference learning on the radial 8-arm maze (RAM) compared to controls. This was accompanied by a significant decrease in memory markers protein kinase Mzeta (PKMζ), calcium impermeable AMPA receptor subunit GluA2, and the post-synaptic density 95 (PSD-95) protein in the hippocampus. Compared to controls, the VOMA paradigm also induced decreases in hippocampal levels of dopamine transporter (DAT) and tyrosine hydroxylase (TH), as well as increases in dopamine 1 receptor (D1R), glial fibrillary acidic protein (GFAP) and cyclooxygenase-2 (COX-2), with a decrease in prostaglandins E2 (PGE2) and D2 (PGD2). These results demonstrate that chronic VOMA reaching 146 mg/kg bw/28d induces significant hippocampal neurotoxicity. Future studies will evaluate the progression of this neurotoxic state.
Keywords: Neuroscience.
Figures




Similar articles
-
Voluntary oral methamphetamine increases memory deficits and contextual sensitization during abstinence associated with decreased PKMζ and increased κOR in the hippocampus of female mice.J Psychopharmacol. 2021 Oct;35(10):1240-1252. doi: 10.1177/02698811211048285. Epub 2021 Sep 29. J Psychopharmacol. 2021. PMID: 34587831 Free PMC article.
-
Synaptic remodeling of GluA1 and GluA2 expression in the nucleus accumbens promotes susceptibility to cognitive deficits concomitant with downstream GSK3β mediated neurotoxicity in female mice during abstinence from voluntary oral methamphetamine.Addict Neurosci. 2023 Dec;8:100112. doi: 10.1016/j.addicn.2023.100112. Epub 2023 Jun 16. Addict Neurosci. 2023. PMID: 37842014 Free PMC article.
-
Methamphetamine-induced short-term increase and long-term decrease in spatial working memory affects protein Kinase M zeta (PKMζ), dopamine, and glutamate receptors.Front Behav Neurosci. 2014 Dec 18;8:438. doi: 10.3389/fnbeh.2014.00438. eCollection 2014. Front Behav Neurosci. 2014. PMID: 25566006 Free PMC article.
-
Detection of methamphetamine neurotoxicity in forensic autopsy cases.Leg Med (Tokyo). 2009 Apr;11 Suppl 1:S63-5. doi: 10.1016/j.legalmed.2009.01.003. Epub 2009 Mar 6. Leg Med (Tokyo). 2009. PMID: 19269222 Review.
-
Methamphetamine induced neurotoxic diseases, molecular mechanism, and current treatment strategies.Biomed Pharmacother. 2022 Oct;154:113591. doi: 10.1016/j.biopha.2022.113591. Epub 2022 Aug 22. Biomed Pharmacother. 2022. PMID: 36007276 Review.
Cited by
-
Voluntary oral methamphetamine increases memory deficits and contextual sensitization during abstinence associated with decreased PKMζ and increased κOR in the hippocampus of female mice.J Psychopharmacol. 2021 Oct;35(10):1240-1252. doi: 10.1177/02698811211048285. Epub 2021 Sep 29. J Psychopharmacol. 2021. PMID: 34587831 Free PMC article.
-
Neuronal and peripheral damages induced by synthetic psychoactive substances: an update of recent findings from human and animal studies.Neural Regen Res. 2020 May;15(5):802-816. doi: 10.4103/1673-5374.268895. Neural Regen Res. 2020. PMID: 31719240 Free PMC article. Review.
-
Extracellular vesicles from mesenchymal stem cells improve neuroinflammation and neurotransmission in hippocampus and cognitive impairment in rats with mild liver damage and minimal hepatic encephalopathy.Stem Cell Res Ther. 2024 Dec 18;15(1):472. doi: 10.1186/s13287-024-04076-6. Stem Cell Res Ther. 2024. PMID: 39696620 Free PMC article.
-
Timapiprant, a prostaglandin D2 receptor antagonist, ameliorates pathology in a rat Alzheimer's model.Life Sci Alliance. 2022 Sep 27;5(12):e202201555. doi: 10.26508/lsa.202201555. Life Sci Alliance. 2022. PMID: 36167438 Free PMC article.
-
Role of Microglia in Psychostimulant Addiction.Curr Neuropharmacol. 2023;21(2):235-259. doi: 10.2174/1570159X21666221208142151. Curr Neuropharmacol. 2023. PMID: 36503452 Free PMC article. Review.
References
-
- Amiry-Moghaddam M., Frydenlund D., Ottersen O. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience. 2004;129(4):997–1008. - PubMed
-
- Battaglia G., Fornai F., Busceti C.L., Aloisi G., Cerrito F., De Blasi A., Melchiorri D., Nicoletti F. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J. Neurosci. 2002;22(6):2135–2141. https://www.ncbi.nlm.nih.gov/pubmed/11896153 - PMC - PubMed
-
- Behr J., Gloveli T., Schmitz D., Heinemann U. Dopamine depresses excitatory synaptic transmission onto rat subicular neurons via presynaptic D1-like dopamine receptors. J. Neurophysiol. 2000;84(1):112–119. https://www.ncbi.nlm.nih.gov/pubmed/10899189 - PubMed
-
- Belcher A.M., Feinstein E.M., O'Dell S.J., Marshall J.F. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33(6):1453–1463. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

