Mefloquine for preventing malaria in pregnant women
- PMID: 29561063
- PMCID: PMC5875065
- DOI: 10.1002/14651858.CD011444.pub2
Mefloquine for preventing malaria in pregnant women
Update in
-
Mefloquine for preventing malaria in pregnant women.Cochrane Database Syst Rev. 2018 Nov 14;11(11):CD011444. doi: 10.1002/14651858.CD011444.pub3. Cochrane Database Syst Rev. 2018. PMID: 30480761 Free PMC article.
Abstract
Background: The World Health Organization recommends intermittent preventive treatment in pregnancy (IPTp) with sulfadoxine-pyrimethamine for malaria for all women who live in moderate to high malaria transmission areas in Africa. However, parasite resistance to sulfadoxine-pyrimethamine has been increasing steadily in some areas of the region. Moreover, HIV-infected women on cotrimoxazole prophylaxis cannot receive sulfadoxine-pyrimethamine because of potential drug interactions. Thus, there is an urgent need to identify alternative drugs for prevention of malaria in pregnancy. One such candidate is mefloquine.
Objectives: To assess the effects of mefloquine for preventing malaria in pregnant women, specifically, to evaluate:• the efficacy, safety, and tolerability of mefloquine for preventing malaria in pregnant women; and• the impact of HIV status, gravidity, and use of insecticide-treated nets on the effects of mefloquine.
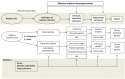
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE, Embase, Latin American Caribbean Health Sciences Literature (LILACS), the Malaria in Pregnancy Library, and two trial registers up to 31 January 2018. In addition, we checked references and contacted study authors to identify additional studies, unpublished data, confidential reports, and raw data from published trials.
Selection criteria: Randomized and quasi-randomized controlled trials comparing mefloquine IPT or mefloquine prophylaxis against placebo, no treatment, or an alternative drug regimen.
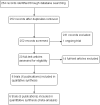
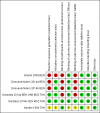
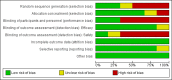
Data collection and analysis: Two review authors independently screened all records identified by the search strategy, applied inclusion criteria, assessed risk of bias, and extracted data. We contacted trial authors to ask for additional information when required. Dichotomous outcomes were compared using risk ratios (RRs), count outcomes as incidence rate ratios (IRRs), and continuous outcomes using mean differences (MDs). We have presented all measures of effect with 95% confidence intervals (CIs). We assessed the certainty of evidence using the GRADE approach for the following main outcomes of analysis: maternal peripheral parasitaemia at delivery, clinical malaria episodes during pregnancy, placental malaria, maternal anaemia at delivery, low birth weight, spontaneous abortions and stillbirths, dizziness, and vomiting.
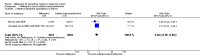
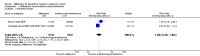
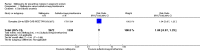
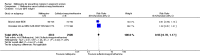
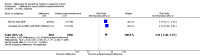
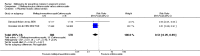
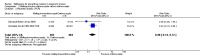
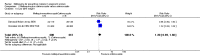
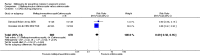
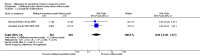
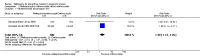
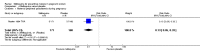
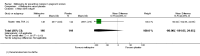
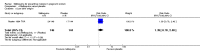
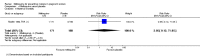
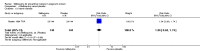
Main results: Six trials conducted between 1987 and 2013 from Thailand (1), Benin (3), Gabon (1), Tanzania (1), Mozambique (2), and Kenya (1) that included 8192 pregnant women met our inclusion criteria.Two trials (with 6350 HIV-uninfected pregnant women) compared two IPTp doses of mefloquine with two IPTp doses of sulfadoxine-pyrimethamine. Two other trials involving 1363 HIV-infected women compared three IPTp doses of mefloquine plus cotrimoxazole with cotrimoxazole. One trial in 140 HIV-infected women compared three doses of IPTp-mefloquine with cotrimoxazole. Finally, one trial enrolling 339 of unknown HIV status compared mefloquine prophylaxis with placebo.Study participants included women of all gravidities and of all ages (four trials) or > 18 years (two trials). Gestational age at recruitment was > 20 weeks (one trial), between 16 and 28 weeks (three trials), or ≤ 28 weeks (two trials). Two of the six trials blinded participants and personnel, and only one had low risk of detection bias for safety outcomes.When compared with sulfadoxine-pyrimethamine, IPTp-mefloquine results in a 35% reduction in maternal peripheral parasitaemia at delivery (RR 0.65, 95% CI 0.48 to 0.86; 5455 participants, 2 studies; high-certainty evidence) but may have little or no effect on placental malaria infections (RR 1.04, 95% CI 0.58 to 1.86; 4668 participants, 2 studies; low-certainty evidence). Mefloquine results in little or no difference in the incidence of clinical malaria episodes during pregnancy (incidence rate ratio (IRR) 0.83, 95% CI 0.65 to 1.05, 2 studies; high-certainty evidence). Mefloquine decreased maternal anaemia at delivery (RR 0.84, 95% CI 0.76 to 0.94; 5469 participants, 2 studies; moderate-certainty evidence). Data show little or no difference in the proportions of low birth weight infants (RR 0.95, 95% CI 0.78 to 1.17; 5641 participants, 2 studies; high-certainty evidence) and in stillbirth and spontaneous abortion rates (RR 1.20, 95% CI 0.91 to 1.58; 6219 participants, 2 studies; I2 statistic = 0%; high-certainty evidence). IPTp-mefloquine increased drug-related vomiting (RR 4.76, 95% CI 4.13 to 5.49; 6272 participants, 2 studies; high-certainty evidence) and dizziness (RR 4.21, 95% CI 3.36 to 5.27; participants = 6272, 2 studies; high-certainty evidence).When compared with cotrimoxazole, IPTp-mefloquine plus cotrimoxazole probably results in a 48% reduction in maternal peripheral parasitaemia at delivery (RR 0.52, 95% CI 0.30 to 0.93; 989 participants, 2 studies; moderate-certainty evidence) and a 72% reduction in placental malaria (RR 0.28, 95% CI 0.14 to 0.57; 977 participants, 2 studies; high-certainty evidence) but has little or no effect on the incidence of clinical malaria episodes during pregnancy (IRR 0.76, 95% CI 0.33 to 1.76, 1 study; high-certainty evidence) and probably no effect on maternal anaemia at delivery (RR 0.94, 95% CI 0.73 to 1.20; 1197 participants, 2 studies; moderate-certainty evidence), low birth weight rates (RR 1.20, 95% CI 0.89 to 1.60; 1220 participants, 2 studies; moderate-certainty evidence), and rates of spontaneous abortion and stillbirth (RR 1.12, 95% CI 0.42 to 2.98; 1347 participants, 2 studies; very low-certainty evidence). Mefloquine was associated with higher risks of drug-related vomiting (RR 7.95, 95% CI 4.79 to 13.18; 1055 participants, one study; high-certainty evidence) and dizziness (RR 3.94, 95% CI 2.85 to 5.46; 1055 participants, 1 study; high-certainty evidence).
Authors' conclusions: Mefloquine was more efficacious than sulfadoxine-pyrimethamine in HIV-uninfected women or daily cotrimoxazole prophylaxis in HIV-infected pregnant women for prevention of malaria infection and was associated with lower risk of maternal anaemia, no adverse effects on pregnancy outcomes (such as stillbirths and abortions), and no effects on low birth weight and prematurity. However, the high proportion of mefloquine-related adverse events constitutes an important barrier to its effectiveness for malaria preventive treatment in pregnant women.
Conflict of interest statement
RG, JJA, and CM are authors of two trials of mefloquine to prevent malaria in pregnancy (published in 2014) that are candidates for inclusion in this review. MP has no known conflicts of interest. CPD has no known conflicts of interest. FtK has no known conflicts of interest.
Figures























































References
References to studies included in this review
-
- Briand V, Bottero J, Noël H, Masse V, Cordel H, Guerra J, et al. Intermittent treatment for the prevention of malaria during pregnancy in Benin: a randomized, open‐label equivalence trial comparing sulfadoxine‐pyrimethamine with mefloquine. Journal of Infectious Diseases 2009;200(6):991‐1001. [DOI: 10.1086/605474] - DOI - PubMed
-
- Denoeud‐Ndam L, Zannou DM, Fourcade C, Taron‐Brocard C, Porcher R, Atadokpede F, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV‐infected pregnant women: two randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes 2014;65(2):198‐206. [DOI: 10.1097/QAI.0000000000000058] - DOI - PubMed
-
- Denoeud‐Ndam L, Zannou DM, Fourcade C, Taron‐Brocard C, Porcher R, Atadokpede F, et al. Cotrimoxazole prophylaxis versus mefloquine intermittent preventive treatment to prevent malaria in HIV‐infected pregnant women: two randomized controlled trials. Journal of Acquired Immune Deficiency Syndromes 2014;65(2):198‐206. [DOI: 10.1097/QAI.0000000000000058] - DOI - PubMed
-
- González R, Mombo‐Ngoma G, Ouédraogo S, Kakolwa MA, Abdulla S, Accrombessi M, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV‐negative women: a multicentre randomized controlled trial. PLoS Medicine 2014;11(9):e1001735. [DOI: 10.1371/journal.pmed.1001733] - DOI - PMC - PubMed
- Rupérez M, González R, Mombo‐Ngoma G, Kabanywanyi AM, Sevene E, Ouédraogo S, et al. Mortality, morbidity, and developmental outcomes in infants born to women who received either mefloquine or sulfadoxine‐pyrimethamine as intermittent preventive treatment of malaria in pregnancy: a cohort study. PLoS Medicine 2016;13(2):e1001964. [DOI: 10.1371/journal.pmed.1001964] - DOI - PMC - PubMed
-
- González R, Mombo‐Ngoma G, Ouédraogo S, Kakolwa MA, Abdulla S, Accrombessi M, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV‐infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo‐controlled trial. PLoS Medicine 2014;11(9):e1001735. [DOI: 10.1371/journal.pmed.1001735] - DOI - PMC - PubMed
References to studies excluded from this review
-
- Balocco R, Bonati M. Mefloquine prophylaxis against malaria for female travellers of childbearing age. Lancet 1992;340(8814):309‐10. - PubMed
-
- Denoeud‐Ndam L, Clément MC, Briand V, Akakpo J, Agossou VK, Atadokpédé F, et al. Tolerability of mefloquine intermittent preventive treatment for malaria in HIV‐infected pregnant women in Benin. Journal of Acquired Immune Deficiency Syndromes 2012;61(1):64‐72. - PubMed
-
- Phillips‐Howard PA, Steffen R, Kerr L, Vanhauwere B, Schildknecht J, Fuchs E, et al. Safety of mefloquine and other antimalarial agents in the first trimester of pregnancy. Journal of Travel Medicine 1998;5(3):121‐6. - PubMed
References to ongoing studies
-
- A comparative study of mefloquine and SP as prophylaxis against malaria in pregnant HIV‐infected patients. Ongoing studySeptember 2015.
Additional references
-
- Ataíde R, Mayor A, Rogerson SJ. Malaria, primigravidae, and antibodies: knowledge gained and future perspectives. Trends in Parasitology 2014;30(2):85‐94. - PubMed
-
- Aubouy A, Fievet N, Bertin G, Sagbo JC, Kossou H, Kinde‐Gazard D, et al. Dramatically decreased therapeutic efficacy of chloroquine and sulfadoxine‐pyrimethamine, but not mefloquine, in southern Benin. Tropical Medicine & International Health 2007;12(7):886‐94. [PUBMED: 17596256] - PubMed
-
- Ayisi JG, Eijk AM, ter Kuile FO, Kolczak MS, Otieno JA, Misore AO, et al. The effect of dual infection with HIV and malaria on pregnancy outcomes in western Kenya. AIDS 2003;17:585‐94. - PubMed
-
- Bardaji A, Bassat Q, Alonso PL, Menendez C. Intermittent preventive treatment of malaria in pregnant women and infants: making best use of the available evidence. Expert Opinion in Pharmacotherapy 2012;13(12):1719‐36. - PubMed
References to other published versions of this review
-
- González R, Boerma RS, Sinclair D, Aponte JJ, ter Kuile FO, Menéndez C. Mefloquine for preventing malaria in pregnant women. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011444] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

