Enantioselective Synthesis of Pyrrolopyrimidine Scaffolds through Cation-Directed Nucleophilic Aromatic Substitution
- PMID: 29561161
- PMCID: PMC5909700
- DOI: 10.1021/acs.orglett.8b00579
Enantioselective Synthesis of Pyrrolopyrimidine Scaffolds through Cation-Directed Nucleophilic Aromatic Substitution
Abstract
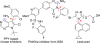
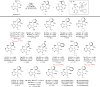
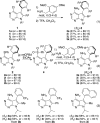
The catalytic enantioselective synthesis of 3-aryl-substituted pyrrolopyrimidines (PPYs), a common motif in drug discovery, is achieved through a kinetic resolution via quaternary ammonium salt-catalyzed nucleophilic aromatic substitution (SNAr). Both enantioenriched products and starting materials can be functionalized with no observed racemization to give enantiodivergent access to diverse chiral analogues of an important class of kinase inhibitor. One of the compounds was found to be a potent and selective inhibitor of breast tumor kinase.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Catalytic enantioselective synthesis of atropisomeric biaryls: a cation-directed nucleophilic aromatic substitution reaction.Angew Chem Int Ed Engl. 2014 Nov 17;53(47):12822-6. doi: 10.1002/anie.201408205. Epub 2014 Sep 24. Angew Chem Int Ed Engl. 2014. PMID: 25257677
-
Stereoelectronic Effects in Ligand Design: Enantioselective Rhodium-Catalyzed Hydrogenation of Aliphatic Cyclic Tetrasubstituted Enamides and Concise Synthesis of (R)-Tofacitinib.Angew Chem Int Ed Engl. 2019 Sep 16;58(38):13573-13583. doi: 10.1002/anie.201908089. Epub 2019 Aug 13. Angew Chem Int Ed Engl. 2019. PMID: 31343811 Review.
-
Chiral aryl-copper(III) electrophiles: new opportunities in catalytic enantioselective arylations and domino processes.Angew Chem Int Ed Engl. 2012 Oct 29;51(44):10934-5. doi: 10.1002/anie.201205805. Epub 2012 Sep 23. Angew Chem Int Ed Engl. 2012. PMID: 23001994
-
Enantioselective 1,3-dipolar cycloaddition reaction between diazoacetates and alpha-substituted acroleins: total synthesis of manzacidin A.J Am Chem Soc. 2006 Feb 22;128(7):2174-5. doi: 10.1021/ja056851u. J Am Chem Soc. 2006. PMID: 16478146
-
Synthetic Entries to and Biological Activity of Pyrrolopyrimidines.Chem Rev. 2016 Jan 13;116(1):80-139. doi: 10.1021/acs.chemrev.5b00483. Epub 2015 Dec 23. Chem Rev. 2016. PMID: 26699634 Review.
Cited by
-
Atropisomerism in the Pharmaceutically Relevant Realm.Acc Chem Res. 2022 Oct 18;55(20):2904-2919. doi: 10.1021/acs.accounts.2c00500. Epub 2022 Sep 26. Acc Chem Res. 2022. PMID: 36153960 Free PMC article.
-
Enantioselective Synthesis of Biaryl Atropisomers via the Addition of Thiophenols into Aryl-Naphthoquinones.ACS Catal. 2018 Jun 1;8(6):5443-5447. doi: 10.1021/acscatal.8b00906. Epub 2018 May 8. ACS Catal. 2018. PMID: 30455999 Free PMC article.
-
Engineered enzymes for enantioselective nucleophilic aromatic substitutions.Nature. 2025 Mar;639(8054):375-381. doi: 10.1038/s41586-025-08611-0. Epub 2025 Jan 15. Nature. 2025. PMID: 39814071 Free PMC article.
-
Dynamic Kinetic Resolution of Indole-Based Sulfenylated Heterobiaryls by Rhodium-Catalyzed Atroposelective Reductive Aldol Reaction.ACS Catal. 2023 Aug 30;13(18):12134-12141. doi: 10.1021/acscatal.3c03422. eCollection 2023 Sep 15. ACS Catal. 2023. PMID: 37745194 Free PMC article.
-
Phase-transfer-catalyst enabled enantioselective C-N coupling to access chiral boron-stereogenic BODIPYs.Nat Commun. 2025 Mar 20;16(1):2735. doi: 10.1038/s41467-025-58117-6. Nat Commun. 2025. PMID: 40108193 Free PMC article.
References
-
- Bringmann G, Price Mortimer AJ, Keller PA, Gresser MJ, Garner J, Breuning M. Angew Chem, Int Ed. 2005;44:5384–5427. - PubMed
-
- Kumarasamy E, Raghunathan R, Sibi MP, Sivaguru J. Chem Rev. 2015;115:11239–11300. - PubMed
-
- Ma G, Sibi MP. Chem – Eur J. 2015;21:11644–11657. - PubMed
-
- Zask A, Murphy J, Ellestad GA. Chirality. 2013;25:265–274. - PubMed
-
- Bringmann G, Gulder T, Gulder TAM, Breuning M. Chem Rev. 2011;111:563–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

