Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes
- PMID: 29561187
- PMCID: PMC6139527
- DOI: 10.1152/ajprenal.00565.2017
Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes
Abstract
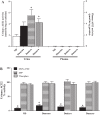
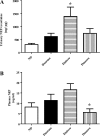
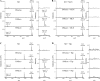
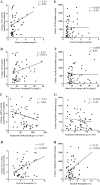
Angiotensin converting enzyme 2 (ACE2) and neprilysin (NEP) are metalloproteases that are highly expressed in the renal proximal tubules. ACE2 and NEP generate renoprotective angiotensin (1-7) from angiotensin II and angiotensin I, respectively, and therefore could have a major role in chronic kidney disease (CKD). Recent data demonstrated increased urinary ACE2 in patients with diabetes with CKD and kidney transplants. We tested the hypothesis that urinary ACE2, NEP, and a disintegrin and metalloproteinase 17 (ADAM17) are increased and could be risk predictors of CKD in patients with diabetes. ACE2, NEP, and ADAM17 were investigated in 20 nondiabetics (ND) and 40 patients with diabetes with normoalbuminuria (Dnormo), microalbuminuria (Dmicro), and macroalbuminuria (Dmacro) using ELISA, Western blot, and fluorogenic and mass spectrometric-based enzyme assays. Logistic regression model was applied to predict the risk prediction. Receiver operating characteristic curves were drawn, and prediction accuracies were calculated to explore the effectiveness of ACE2 and NEP in predicting diabetes and CKD. Results demonstrated that there is no evidence of urinary ACE2 and ADAM17 in ND subjects, but both enzymes were increased in patients with diabetes, including Dnormo. Although there was no detectable plasma ACE2 activity, there was evidence of urinary and plasma NEP in all the subjects, and urinary NEP was significantly increased in Dmicro patients. NEP and ACE2 showed significant correlations with metabolic and renal characteristics. In summary, urinary ACE2, NEP, and ADAM17 are increased in patients with diabetes and could be used as early biomarkers to predict the incidence or progression of CKD at early stages among individuals with type 2 diabetes.
Keywords: ACE2; ADAM17; NEP; chronic kidney disease; diabetic nephropathy; type 2 diabetes.
Figures

Similar articles
-
Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion.PLoS One. 2013 Apr 30;8(4):e62833. doi: 10.1371/journal.pone.0062833. Print 2013. PLoS One. 2013. PMID: 23646149 Free PMC article.
-
Daily exercise training protects against albuminuria and angiotensin converting enzyme 2 shedding in db/db diabetic mice.J Endocrinol. 2014 Apr 22;221(2):235-51. doi: 10.1530/JOE-13-0532. Print 2014 May. J Endocrinol. 2014. PMID: 24756098 Free PMC article.
-
Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice.Am J Physiol Renal Physiol. 2014 Mar 15;306(6):F629-39. doi: 10.1152/ajprenal.00516.2013. Epub 2014 Jan 22. Am J Physiol Renal Physiol. 2014. PMID: 24452639 Free PMC article.
-
A review of urinary angiotensin converting enzyme 2 in diabetes and diabetic nephropathy.Biochem Med (Zagreb). 2019 Feb 15;29(1):010501. doi: 10.11613/BM.2019.010501. Epub 2018 Dec 15. Biochem Med (Zagreb). 2019. PMID: 30591810 Free PMC article. Review.
-
Role of ADAM17 in kidney disease.Am J Physiol Renal Physiol. 2019 Aug 1;317(2):F333-F342. doi: 10.1152/ajprenal.00625.2018. Epub 2019 May 29. Am J Physiol Renal Physiol. 2019. PMID: 31141400 Review.
Cited by
-
Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19).Diabetes Metab Res Rev. 2021 Feb;37(2):e3377. doi: 10.1002/dmrr.3377. Epub 2020 Jul 20. Diabetes Metab Res Rev. 2021. PMID: 32588943 Free PMC article. Review.
-
Stimulation of Angiotensin Converting Enzyme 2: A Novel Treatment Strategy for Diabetic Nephropathy.Front Physiol. 2022 Jan 11;12:813012. doi: 10.3389/fphys.2021.813012. eCollection 2021. Front Physiol. 2022. PMID: 35087423 Free PMC article. Review.
-
Neprilysin expression and functions in development, ageing and disease.Mech Ageing Dev. 2020 Dec;192:111363. doi: 10.1016/j.mad.2020.111363. Epub 2020 Sep 26. Mech Ageing Dev. 2020. PMID: 32987038 Free PMC article. Review.
-
Determination of soluble angiotensin-converting enzyme 2 in saliva samples and its association with nicotine.Environ Res. 2023 Jan 1;216(Pt 1):114443. doi: 10.1016/j.envres.2022.114443. Epub 2022 Oct 3. Environ Res. 2023. PMID: 36195157 Free PMC article.
-
Angiotensin-converting enzyme 2 and COVID-19: patients, comorbidities, and therapies.Am J Physiol Lung Cell Mol Physiol. 2021 Mar 1;320(3):L301-L330. doi: 10.1152/ajplung.00259.2020. Epub 2020 Nov 25. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 33237815 Free PMC article. Review.
References
-
- Burns KD, Lytvyn Y, Mahmud FH, Daneman D, Deda L, Dunger DB, Deanfield J, Dalton RN, Elia Y, Har R, Van JAD, Bradley TJ, Slorach C, Hui W, Xiao F, Zimpelmann J, Mertens L, Moineddin R, Reich HN, Sochett E, Scholey JW, Cherney DZI. The relationship between urinary renin-angiotensin system markers, renal function, and blood pressure in adolescents with type 1 diabetes. Am J Physiol Renal Physiol 312: F335–F342, 2017. doi:10.1152/ajprenal.00438.2016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

