A role for FGF2 in visceral adiposity-associated mammary epithelial transformation
- PMID: 29561195
- PMCID: PMC6152512
- DOI: 10.1080/21623945.2018.1445889
A role for FGF2 in visceral adiposity-associated mammary epithelial transformation
Abstract
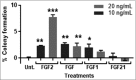
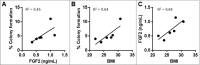
Obesity is a leading risk factor for post-menopausal breast cancer, and this is concerning as 40% of cancer diagnoses in 2014 were associated with overweight/obesity. Despite this epidemiological link, the underlying mechanism responsible is unknown. We recently published that visceral adipose tissue (VAT) releases FGF2 and stimulates the transformation of skin epithelial cells. Furthermore, obesity is differentially associated with many epithelial cancers, and this mechanistic link could be translational. As FGF2 and FGFR1 are implicated in breast cancer progression, we hypothesize that VAT-derived FGF2 plays a translational role in promoting adiposity-associated mammary epithelial cell transformation. In this brief report, data suggest that FGF2/FGFR1 signaling is a potential mechanistic link in VAT-stimulated transformation of breast epithelial cells.
Keywords: FGF2; Obesity; adiposity; breast; breast cancer; cancer; visceral fat.
Figures


Similar articles
-
Fibroblast growth factor receptor is a mechanistic link between visceral adiposity and cancer.Oncogene. 2017 Nov 30;36(48):6668-6679. doi: 10.1038/onc.2017.278. Epub 2017 Aug 7. Oncogene. 2017. PMID: 28783178 Free PMC article.
-
Elucidating the role of adipose tissue secreted factors in malignant transformation.Adipocyte. 2018 Jan 2;7(1):45-48. doi: 10.1080/21623945.2017.1388971. Epub 2017 Nov 2. Adipocyte. 2018. PMID: 29095087 Free PMC article.
-
The Use of Human Serum Samples to Study Malignant Transformation: A Pilot Study.Cells. 2021 Oct 6;10(10):2670. doi: 10.3390/cells10102670. Cells. 2021. PMID: 34685650 Free PMC article.
-
Visceral adiposity and cancer survival: a review of imaging studies.Eur J Cancer Care (Engl). 2018 Mar;27(2):e12611. doi: 10.1111/ecc.12611. Epub 2016 Dec 6. Eur J Cancer Care (Engl). 2018. PMID: 27921375 Review.
-
Stop feeding cancer: pro-inflammatory role of visceral adiposity in liver cancer.Cytokine. 2013 Dec;64(3):626-37. doi: 10.1016/j.cyto.2013.09.009. Epub 2013 Oct 11. Cytokine. 2013. PMID: 24120848 Review.
Cited by
-
Identifying chemopreventive agents for obesity-associated cancers using an efficient, 3D high-throughput transformation assay.Sci Rep. 2019 Jul 16;9(1):10278. doi: 10.1038/s41598-019-46531-y. Sci Rep. 2019. PMID: 31311976 Free PMC article.
-
A risk-associated Active transcriptome phenotype expressed by histologically normal human breast tissue and linked to a pro-tumorigenic adipocyte population.Breast Cancer Res. 2020 Jul 31;22(1):81. doi: 10.1186/s13058-020-01322-6. Breast Cancer Res. 2020. PMID: 32736587 Free PMC article.
-
Visceral Obesity and Its Shared Role in Cancer and Cardiovascular Disease: A Scoping Review of the Pathophysiology and Pharmacological Treatments.Int J Mol Sci. 2020 Nov 27;21(23):9042. doi: 10.3390/ijms21239042. Int J Mol Sci. 2020. PMID: 33261185 Free PMC article.
-
Inflammasome activation as a link between obesity and thyroid disorders: Implications for an integrated clinical management.Front Endocrinol (Lausanne). 2022 Aug 19;13:959276. doi: 10.3389/fendo.2022.959276. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060941 Free PMC article. Review.
-
PTEN: A Thrifty Gene That Causes Disease in Times of Plenty?Front Nutr. 2020 Jun 5;7:81. doi: 10.3389/fnut.2020.00081. eCollection 2020. Front Nutr. 2020. PMID: 32582754 Free PMC article.
References
-
- Huang Z, Willett W, Coldfe G, et al.. Waist circumference, waist:hip ratio, and risk of breast cancer in the nurses' health study. Am J Epidemiol. 1999. 04/08/1999;150(2):8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
