HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations
- PMID: 29561264
- PMCID: PMC5896952
- DOI: 10.7554/eLife.34271
HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations
Abstract
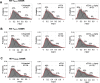
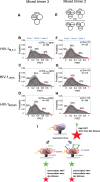
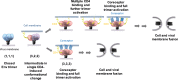
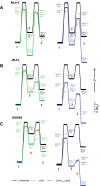
HIV-1 entry into cells requires binding of the viral envelope glycoprotein (Env) to receptor CD4 and coreceptor. Imaging of individual Env molecules on native virions shows Env trimers to be dynamic, spontaneously transitioning between three distinct well-populated conformational states: a pre-triggered Env (State 1), a default intermediate (State 2) and a three-CD4-bound conformation (State 3), which can be stabilized by binding of CD4 and coreceptor-surrogate antibody 17b. Here, using single-molecule Fluorescence Resonance Energy Transfer (smFRET), we show the default intermediate configuration to be asymmetric, with individual protomers adopting distinct conformations. During entry, this asymmetric intermediate forms when a single CD4 molecule engages the trimer. The trimer can then transition to State 3 by binding additional CD4 molecules and coreceptor.
Keywords: HIV-1 Envelope; Single molecule FRET; infectious disease; microbiology; molecular biophysics; structural biology; virus; virus entry.
Conflict of interest statement
XM, ML, JG, DT, XH, ZZ, HZ, RA, JA, SB, PK, JM No competing interests declared, WM Patent applications pertaining to this work are U.S. Patent Application 13/202,351, Methods and Compositions for Altering Photophysical Properties of Fluorophores via Proximal Quenching (S.C.B., Z.Z.); U.S. Patent Application 14/373,402 Dye Compositions, Methods of Preparation, Conjugates Thereof, and Methods of Use (S.C.B., Z.Z.); and International and US Patent Application PCT/US13/42249 Reagents and Methods for Identifying Anti-HIV Compounds (S.C.B., J.B.M., W.M.). S.C.B. is a co-founder of Lumidyne Corporation
Figures








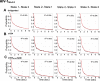
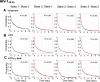
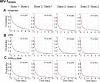
Similar articles
-
Shedding-Resistant HIV-1 Envelope Glycoproteins Adopt Downstream Conformations That Remain Responsive to Conformation-Preferring Ligands.J Virol. 2020 Aug 17;94(17):e00597-20. doi: 10.1128/JVI.00597-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32522853 Free PMC article.
-
Activation and Inactivation of Primary Human Immunodeficiency Virus Envelope Glycoprotein Trimers by CD4-Mimetic Compounds.J Virol. 2017 Jan 18;91(3):e01880-16. doi: 10.1128/JVI.01880-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881646 Free PMC article.
-
SOSIP Changes Affect Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Conformation and CD4 Engagement.J Virol. 2018 Sep 12;92(19):e01080-18. doi: 10.1128/JVI.01080-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021898 Free PMC article.
-
Quaternary Interaction of the HIV-1 Envelope Trimer with CD4 and Neutralizing Antibodies.Viruses. 2021 Jul 20;13(7):1405. doi: 10.3390/v13071405. Viruses. 2021. PMID: 34372611 Free PMC article. Review.
-
Conformation-Dependent Interactions Between HIV-1 Envelope Glycoproteins and Broadly Neutralizing Antibodies.AIDS Res Hum Retroviruses. 2018 Sep;34(9):794-803. doi: 10.1089/AID.2018.0102. Epub 2018 Jul 17. AIDS Res Hum Retroviruses. 2018. PMID: 29905080 Review.
Cited by
-
HIV-1 Envelope Conformation, Allostery, and Dynamics.Viruses. 2021 May 7;13(5):852. doi: 10.3390/v13050852. Viruses. 2021. PMID: 34067073 Free PMC article. Review.
-
Conformational Differences between Functional Human Immunodeficiency Virus Envelope Glycoprotein Trimers and Stabilized Soluble Trimers.J Virol. 2019 Jan 17;93(3):e01709-18. doi: 10.1128/JVI.01709-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429345 Free PMC article.
-
Temsavir blocks the immunomodulatory activities of HIV-1 soluble gp120.Cell Chem Biol. 2023 May 18;30(5):540-552.e6. doi: 10.1016/j.chembiol.2023.03.003. Epub 2023 Mar 22. Cell Chem Biol. 2023. PMID: 36958337 Free PMC article.
-
Cryo-EM Structure of Full-length HIV-1 Env Bound With the Fab of Antibody PG16.J Mol Biol. 2020 Feb 14;432(4):1158-1168. doi: 10.1016/j.jmb.2019.11.028. Epub 2020 Jan 11. J Mol Biol. 2020. PMID: 31931014 Free PMC article.
-
Inhibition of human immunodeficiency virus (HIV-1) infectivity by expression of poorly or broadly neutralizing antibodies against Env in virus-producing cells.J Virol. 2024 Feb 20;98(2):e0159423. doi: 10.1128/jvi.01594-23. Epub 2024 Jan 30. J Virol. 2024. PMID: 38289101 Free PMC article.
References
-
- Arthos J, Cicala C, Steenbeke TD, Chun TW, Dela Cruz C, Hanback DB, Khazanie P, Nam D, Schuck P, Selig SM, Van Ryk D, Chaikin MA, Fauci AS. Biochemical and biological characterization of a dodecameric CD4-Ig fusion protein: implications for therapeutic and vaccine strategies. The Journal of Biological Chemistry. 2002;277:11456–11464. doi: 10.1074/jbc.M111191200. - DOI - PubMed
-
- Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fätkenheuer G, Schlesinger SJ, Nussenzweig MC. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI005023-17/NH/NIH HHS/United States
- R01 GM098859/GM/NIGMS NIH HHS/United States
- GM098859/NH/NIH HHS/United States
- R01 GM116654/GM/NIGMS NIH HHS/United States
- P01 GM056550/GM/NIGMS NIH HHS/United States
- AI116262/NH/NIH HHS/United States
- GM103310/NH/NIH HHS/United States
- GM116654/NH/NIH HHS/United States
- P30 AI042853/AI/NIAID NIH HHS/United States
- K22 AI116262/AI/NIAID NIH HHS/United States
- Z01 AI005023/ImNIH/Intramural NIH HHS/United States
- P41 GM103310/GM/NIGMS NIH HHS/United States
- AI042853/NH/NIH HHS/United States
- GM056550/NH/NIH HHS/United States
- Irvington Fellows Program/CRI/Cancer Research Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

