Potential clinical and immunotherapeutic utility of talimogene laherparepvec for patients with melanoma after disease progression on immune checkpoint inhibitors and BRAF inhibitors
- PMID: 29561296
- PMCID: PMC5929488
- DOI: 10.1097/CMR.0000000000000444
Potential clinical and immunotherapeutic utility of talimogene laherparepvec for patients with melanoma after disease progression on immune checkpoint inhibitors and BRAF inhibitors
Abstract
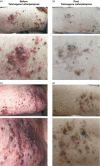
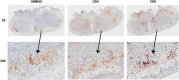
Talimogene laherparepvec is a genetically modified herpes simplex virus type 1-based oncolytic immunotherapy for the local treatment of unresectable subcutaneous and nodal tumors in patients with melanoma recurrent after initial surgery. We report on two patients with melanoma who, after progression on numerous systemic therapies, derived clinical benefit from talimogene laherparepvec in an expanded-access protocol (ClinicalTrials.gov, NCT02147951). Intralesional talimogene laherparepvec (day 1, ≤4 ml 10 PFU/ml; after 3 weeks, ≤4 ml 10 PFU/ml every 2 weeks) was administered until complete response, no injectable tumors, progressive disease, or intolerance occurred. Patient 1 was 71 years old, had stage IIIB disease, and had previously received granulocyte-macrophage colony-stimulating factor, vemurafenib, metformin, ipilimumab, dabrafenib, trametinib, and pembrolizumab. Patient 2 was 45 years old, had stage IIIC disease, and had previously received nivolumab/ipilimumab combination therapy. There were marked reductions in the number and size of melanoma lesions during treatment with talimogene laherparepvec. Both patients experienced mild-to-moderate nausea and vomiting, which were managed using ondansetron, metoclopramide, and pantoprazole. Both patients completed treatment with talimogene laherparepvec in the expanded-access protocol on 24 November 2015, but received talimogene laherparepvec in clinical practice. Patient 1 continues to receive therapy (>60 weeks); patient 2 experienced a complete response at 23 weeks. Immunohistochemistry of a biopsied dermal metastasis from patient 1 showed a marked infiltration of CD4 and CD8 T cells after 1 year of treatment. Talimogene laherparepvec was active in patients with advanced melanoma with disease progression following multiple previous systemic therapies; no new safety signals were identified.
Figures
Similar articles
-
Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma.Melanoma Res. 2018 Feb;28(1):44-51. doi: 10.1097/CMR.0000000000000399. Melanoma Res. 2018. PMID: 29176501 Clinical Trial.
-
The safety of talimogene laherparepvec for the treatment of advanced melanoma.Expert Opin Drug Saf. 2017 Feb;16(2):265-269. doi: 10.1080/14740338.2017.1274729. Epub 2016 Dec 28. Expert Opin Drug Saf. 2017. PMID: 27989216 Review.
-
Talimogene Laherparepvec: A Review in Unresectable Metastatic Melanoma.BioDrugs. 2016 Oct;30(5):461-468. doi: 10.1007/s40259-016-0189-y. BioDrugs. 2016. PMID: 27516203 Review.
-
Real-World Use of Talimogene Laherparepvec in German Patients with Stage IIIB to IVM1a Melanoma: A Retrospective Chart Review and Physician Survey.Adv Ther. 2019 Jan;36(1):101-117. doi: 10.1007/s12325-018-0850-6. Epub 2018 Dec 7. Adv Ther. 2019. PMID: 30536143 Free PMC article.
-
Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors.Cancer Immunol Immunother. 2017 Jun;66(6):683-695. doi: 10.1007/s00262-017-1967-1. Epub 2017 Feb 25. Cancer Immunol Immunother. 2017. PMID: 28238174 Free PMC article. Review.
Cited by
-
Talimogene laherparepvec treatment to overcome loco-regional acquired resistance to immune checkpoint blockade in tumor stage IIIB-IV M1c melanoma patients.Cancer Immunol Immunother. 2020 May;69(5):759-769. doi: 10.1007/s00262-020-02487-x. Epub 2020 Feb 12. Cancer Immunol Immunother. 2020. PMID: 32052079 Free PMC article.
-
Insights into the Molecular Mechanisms Behind Intralesional Immunotherapies for Advanced Melanoma.Cancers (Basel). 2020 May 22;12(5):1321. doi: 10.3390/cancers12051321. Cancers (Basel). 2020. PMID: 32455916 Free PMC article. Review.
-
Combination of pembrolizumab and imatinib in a patient with double KIT mutant melanoma: A case report.Medicine (Baltimore). 2019 Nov;98(44):e17769. doi: 10.1097/MD.0000000000017769. Medicine (Baltimore). 2019. PMID: 31689840 Free PMC article.
-
Combination of Immunotherapy With Targeted Therapy: Theory and Practice in Metastatic Melanoma.Front Immunol. 2019 May 7;10:990. doi: 10.3389/fimmu.2019.00990. eCollection 2019. Front Immunol. 2019. PMID: 31134073 Free PMC article. Review.
-
Observational study of talimogene laherparepvec use in the anti-PD-1 era for melanoma in the US (COSMUS-2).Melanoma Manag. 2020 Jun 15;7(2):MMT41. doi: 10.2217/mmt-2020-0005. Melanoma Manag. 2020. PMID: 32821373 Free PMC article.
References
-
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358–365. - PubMed
-
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015; 386:444–451. - PubMed
-
- Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867–1876. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

