Polarization Variability in Age-related Macular Degeneration
- PMID: 29561503
- PMCID: PMC6688506
- DOI: 10.1097/OPX.0000000000001197
Polarization Variability in Age-related Macular Degeneration
Abstract
Significance: Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss. Complementary imaging techniques can be used to better characterize and quantify pathological changes associated with AMD. By assessing specific light-tissue interactions, polarization-sensitive imaging can be used to detect tissue disruption early in the disease process.
Purpose: The aim of this study was to compare variability in central macular polarization properties in patients with nonexudative AMD and age-matched control subjects.
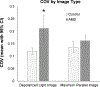
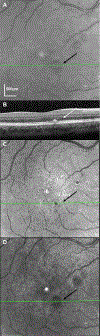
Methods: A scanning laser polarimeter (GDx, LDT/CZM) was used to acquire 15 × 15-degree macular images in 10 subjects diagnosed with nonexudative AMD and 10 age-matched control subjects. The coefficient of variation (COV, SD/mean) was used to quantify variability in pixel intensity in the central 3.3° of the macula for custom images emphasizing multiply scattered light (the depolarized light image) and polarization-retaining light (the maximum of the parallel detector image). The intensity COV was compared across subject categories using paired t tests for each image type.
Results: The COV in the central macula was significantly higher in the AMD subject group (average, 0.221; 95% confidence interval [CI], 0.157 to 0.265) when compared with matched control subjects (average 0.120; 95% CI, 0.107 to 0.133) in the depolarized light image (P = .01). The COV in the maximum of the parallel detector image was not statistically different between the two subject groups (AMD average, 0.162 [95% CI, 0.138 to 0.185]; control average, 0.137 [95% CI, 0.115 to 0.158]; P = .21).
Conclusions: Variability in multiply scattered light is higher than that of light that is more polarization preserving in patients with nonexudative AMD. Multiple scattering may act as an early indicator representing disruption to the macula in early AMD.
Figures







Similar articles
-
Henle fiber layer phase retardation changes associated with age-related macular degeneration.Invest Ophthalmol Vis Sci. 2014 Dec 18;56(1):284-90. doi: 10.1167/iovs.14-14459. Invest Ophthalmol Vis Sci. 2014. PMID: 25525166 Free PMC article.
-
Foveal localization in non-exudative AMD using scanning laser polarimetry.Optom Vis Sci. 2012 May;89(5):667-77. doi: 10.1097/OPX.0b013e31824eeb25. Optom Vis Sci. 2012. PMID: 22466102 Free PMC article.
-
Evaluation of intraretinal migration of retinal pigment epithelial cells in age-related macular degeneration using polarimetric imaging.Sci Rep. 2017 Jun 9;7(1):3150. doi: 10.1038/s41598-017-03529-8. Sci Rep. 2017. PMID: 28600515 Free PMC article.
-
Nonexudative Macular Neovascularization - A Systematic Review of Prevalence, Natural History, and Recent Insights from OCT Angiography.Ophthalmol Retina. 2020 Jul;4(7):651-661. doi: 10.1016/j.oret.2020.02.016. Epub 2020 Mar 13. Ophthalmol Retina. 2020. PMID: 32335033 Free PMC article.
-
Anatomic Clinical Trial Endpoints for Nonexudative Age-Related Macular Degeneration.Ophthalmology. 2016 May;123(5):1060-79. doi: 10.1016/j.ophtha.2016.01.034. Epub 2016 Mar 5. Ophthalmology. 2016. PMID: 26952592 Review.
Cited by
-
Cones in ageing and harsh environments: the neural economy hypothesis.Ophthalmic Physiol Opt. 2020 Mar;40(2):88-116. doi: 10.1111/opo.12670. Epub 2020 Feb 4. Ophthalmic Physiol Opt. 2020. PMID: 32017191 Free PMC article. Review.
-
Near-infrared polarimetric imaging and changes associated with normative aging.J Opt Soc Am A Opt Image Sci Vis. 2018 Sep 1;35(9):1487-1495. doi: 10.1364/JOSAA.35.001487. J Opt Soc Am A Opt Image Sci Vis. 2018. PMID: 30183002 Free PMC article.
-
Three-Dimensional Distribution Of Fundus Depolarization and Associating Factors Measured Using Polarization-Sensitive Optical Coherence Tomography.Transl Vis Sci Technol. 2021 Feb 5;10(2):30. doi: 10.1167/tvst.10.2.30. Transl Vis Sci Technol. 2021. PMID: 34003915 Free PMC article.
-
Foveal Phase Retardation Correlates With Optically Measured Henle Fiber Layer Thickness.Front Med (Lausanne). 2022 Apr 15;9:846738. doi: 10.3389/fmed.2022.846738. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35492328 Free PMC article.
-
Gallic Acid-Encapsulated PAMAM Dendrimers as an Antioxidant Delivery System for Controlled Release and Reduced Cytotoxicity against ARPE-19 Cells.Bioconjug Chem. 2024 Dec 18;35(12):1959-1969. doi: 10.1021/acs.bioconjchem.4c00475. Epub 2024 Dec 6. Bioconjug Chem. 2024. PMID: 39641479 Free PMC article.
References
-
- Freeman SR, Kozak I, Cheng L, et al. Optical Coherence Tomography-Raster Scanning and Manual Segmentation in Determining Drusen Volume in Age-related Macular Degeneration. Retina 2010;30:431–5. - PubMed
-
- Zweifel SA, Imamura Y, Spaide TC, et al. Prevalence and Significance of Subretinal Drusenoid Deposits (Reticular Pseudodrusen) in Age-related Macular Degeneration. Ophthalmology 2010;117:1775–81. - PubMed
-
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A Role for Local Inflammation in the Formation of Drusen in the Aging Eye. Am J Ophthalmol 2002;134:411–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

