Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: the origin of the bimodal size distribution
- PMID: 29561559
- PMCID: PMC6637654
- DOI: 10.1039/C7NR08614H
Two mechanisms of nanoparticle generation in picosecond laser ablation in liquids: the origin of the bimodal size distribution
Abstract
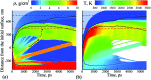
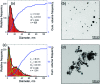
The synthesis of chemically clean and environmentally friendly nanoparticles through pulsed laser ablation in liquids has shown a number of advantages over conventional chemical synthesis methods and has evolved into a thriving research field attracting laboratory and industrial applications. The fundamental understanding of processes leading to the nanoparticle generation, however, still remains elusive. In particular, the origin of bimodal nanoparticle size distributions in femto- and picosecond laser ablation in liquids, where small nanoparticles (several nanometers) with narrow size distribution are commonly observed to coexist with larger (tens to hundreds of nanometers) ones, has not been explained so far. In this paper, joint computational and experimental efforts are applied to understand the mechanisms of nanoparticle formation in picosecond laser ablation in liquids and to explain the bimodal nanoparticle size distributions. The results of a large-scale atomistic simulation reveal the critical role of the dynamic interaction between the ablation plume and the liquid environment, leading to the generation of large nanoparticles through a sequence of hydrodynamic instabilities at the plume-liquid interface and a concurrent nucleation and growth of small nanoparticles in an expanding metal-liquid mixing region. The computational predictions are supported by a series of stroboscopic videography experiments showing the emergence of small satellite bubbles surrounding the main cavitation bubble generated in single pulse experiments. Carefully timed double pulse irradiation triggers expansion of secondary cavitation bubbles indicating, in accord with the simulation results, the presence of localized sites of laser energy deposition (possibly large nanoparticles) injected into the liquid at the early stage of the bubble formation.
Figures



References
-
- Barcikowski S., Devesa F., Moldenhauer K. J. Nanopart. Res. 2009;11:1883–1893.
-
- Amendola V., Meneghetti M. Phys. Chem. Chem. Phys. 2009;11:3805–3821. - PubMed
-
- Zhang D., Gökce B., Barcikowski S. Chem. Rev. 2017;117:3990–4103. - PubMed
-
- Streubel R., Barcikowski S., Gökce B. Opt. Lett. 2016;41:1486–1489. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

