Social status affects lipid metabolism in rainbow trout, Oncorhynchus mykiss
- PMID: 29561648
- PMCID: PMC6139616
- DOI: 10.1152/ajpregu.00402.2017
Social status affects lipid metabolism in rainbow trout, Oncorhynchus mykiss
Abstract
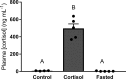
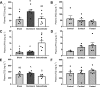
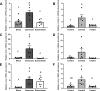
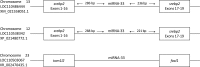
Juvenile rainbow trout ( Oncorhynchus mykiss) confined in pairs form social hierarchies in which socially subordinate fish display characteristic traits, including reduced growth rates and altered glucose metabolism. These effects are, in part, mediated by chronically elevated cortisol levels and/or reduced feeding. To determine the effects of social status on lipid metabolism, trout were held in pairs for 4 days, following which organismal and liver-specific indexes of lipid metabolism were measured. At the organismal level, circulating triglycerides were elevated in dominant trout, whereas subordinate trout exhibited elevated concentrations of circulating free fatty acids (FFAs) and lowered plasma total cholesterol levels. At the molecular level, increased expression of lipogenic genes in dominant trout and cpt1a in subordinate trout was identified, suggesting a contribution of increased de novo lipogenesis to circulating triglycerides in dominant trout and reliance on circulating FFAs for β-oxidation in the liver of subordinates. Given the emerging importance of microRNAs (miRNA) in the regulation of hepatic lipid metabolism, candidate miRNAs were profiled, revealing increased expression of the lipogenic miRNA-33 in dominant fish. Because the Akt-TOR-S6-signaling pathway is an important upstream regulator of hepatic lipid metabolism, its signaling activity was quantified. However, the only difference detected among groups was a strong increase in S6 phosphorylation in subordinate trout. In general, the changes observed in lipid metabolism of subordinates were not mimicked by either cortisol treatment or fasting alone, indicating the existence of specific, emergent effects of subordinate social status itself on this fuel.
Keywords: behavior; cell signaling; energy metabolism; gene expression; hierarchy; liver, microRNA; salmonid.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

