Neural Mechanisms of Social Cognition in Primates
- PMID: 29561702
- PMCID: PMC7116801
- DOI: 10.1146/annurev-neuro-080317-061450
Neural Mechanisms of Social Cognition in Primates
Abstract
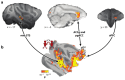
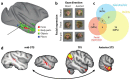
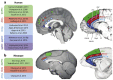
Activity in a network of areas spanning the superior temporal sulcus, dorsomedial frontal cortex, and anterior cingulate cortex is concerned with how nonhuman primates negotiate the social worlds in which they live. Central aspects of these circuits are retained in humans. Activity in these areas codes for primates' interactions with one another, their attempts to find out about one another, and their attempts to prevent others from finding out too much about themselves. Moreover, important features of the social world, such as dominance status, cooperation, and competition, modulate activity in these areas. We consider the degree to which activity in these regions is simply encoding an individual's own actions and choices or whether this activity is especially and specifically concerned with social cognition. Recent advances in comparative anatomy and computational modeling may help us to gain deeper insights into the nature and boundaries of primate social cognition.
Keywords: cingulate cortex; dominance; dorsomedial prefrontal cortex; social network; superior temporal sulcus.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

