Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection
- PMID: 29561727
- PMCID: PMC5862523
- DOI: 10.7554/eLife.34861
Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection
Abstract
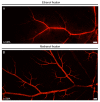
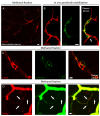
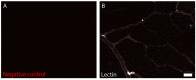
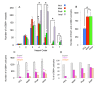
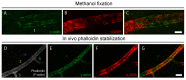
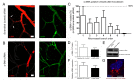
Recent evidence suggests that capillary pericytes are contractile and play a crucial role in the regulation of microcirculation. However, failure to detect components of the contractile apparatus in capillary pericytes, most notably α-smooth muscle actin (α-SMA), has questioned these findings. Using strategies that allow rapid filamentous-actin (F-actin) fixation (i.e. snap freeze fixation with methanol at -20°C) or prevent F-actin depolymerization (i.e. with F-actin stabilizing agents), we demonstrate that pericytes on mouse retinal capillaries, including those in intermediate and deeper plexus, express α-SMA. Junctional pericytes were more frequently α-SMA-positive relative to pericytes on linear capillary segments. Intravitreal administration of short interfering RNA (α-SMA-siRNA) suppressed α-SMA expression preferentially in high order branch capillary pericytes, confirming the existence of a smaller pool of α-SMA in distal capillary pericytes that is quickly lost by depolymerization. We conclude that capillary pericytes do express α-SMA, which rapidly depolymerizes during tissue fixation thus evading detection by immunolabeling.
Keywords: F-actin; alpha-smooth muscle actin; capillary; mouse; neuroscience; retinal pericytes.
© 2018, Alarcon-Martinez et al.
Conflict of interest statement
LA, SY, MY, JS, KK, AC, AD, TD No competing interests declared
Figures



References
-
- Arnold TD, Ferrero GM, Qiu H, Phan IT, Akhurst RJ, Huang EJ, Reichardt LF. Defective retinal vascular endothelial cell development as a consequence of impaired integrin αVβ8-mediated activation of transforming growth factor-β. Journal of Neuroscience. 2012;32:1197–1206. doi: 10.1523/JNEUROSCI.5648-11.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

