Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells
- PMID: 29561796
- PMCID: PMC5979503
- DOI: 10.3390/ijms19040936
Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells
Abstract
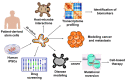
The rising interest in human induced pluripotent stem cell (hiPSC)-derived organoid culture has stemmed from the manipulation of various combinations of directed multi-lineage differentiation and morphogenetic processes that mimic organogenesis. Organoids are three-dimensional (3D) structures that are comprised of multiple cell types, self-organized to recapitulate embryonic and tissue development in vitro. This model has been shown to be superior to conventional two-dimensional (2D) cell culture methods in mirroring functionality, architecture, and geometric features of tissues seen in vivo. This review serves to highlight recent advances in the 3D organoid technology for use in modeling complex hereditary diseases, cancer, host-microbe interactions, and possible use in translational and personalized medicine where organoid cultures were used to uncover diagnostic biomarkers for early disease detection via high throughput pharmaceutical screening. In addition, this review also aims to discuss the advantages and shortcomings of utilizing organoids in disease modeling. In summary, studying human diseases using hiPSC-derived organoids may better illustrate the processes involved due to similarities in the architecture and microenvironment present in an organoid, which also allows drug responses to be properly recapitulated in vitro.
Keywords: 3D organoids; CRISPR/Cas-9; disease modeling; drug screening; genome editing; hereditary diseases; human induced pluripotent stem cells; infectious diseases; microenvironment; neurodevelopmental disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Firth A.L., Menon T., Parker G.S., Qualls S.J., Lewis B.M., Ke E., Dargitz C.T., Wright R., Khanna A., Gage F.H., et al. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient ipscs. Cell Rep. 2015;12:1385–1390. doi: 10.1016/j.celrep.2015.07.062. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources