The Role of microRNAs in Alzheimer's Disease and Their Therapeutic Potentials
- PMID: 29561798
- PMCID: PMC5924516
- DOI: 10.3390/genes9040174
The Role of microRNAs in Alzheimer's Disease and Their Therapeutic Potentials
Abstract
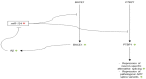
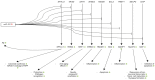
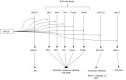
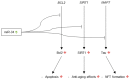
MicroRNAs (miRNAs) are short, endogenous, non-coding RNAs that post-transcriptionally regulate gene expression by base pairing with mRNA targets. Altered miRNA expression profiles have been observed in several diseases, including neurodegeneration. Multiple studies have reported altered expressions of miRNAs in the brains of individuals with Alzheimer's disease (AD) as compared to those of healthy elderly adults. Some of the miRNAs found to be dysregulated in AD have been reported to correlate with neuropathological changes, including plaque and tangle accumulation, as well as altered expressions of species that are known to be involved in AD pathology. To examine the potentially pathogenic functions of several dysregulated miRNAs in AD, we review the current literature with a focus on the activities of ten miRNAs in biological pathways involved in AD pathogenesis. Comprehensive understandings of the expression profiles and activities of these miRNAs will illuminate their roles as potential therapeutic targets in AD brain and may lead to the discovery of breakthrough treatment strategies for AD.
Keywords: Alzheimer’s disease; BACE1 inhibitors; miRNAs; γ-secretase inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
-
- Jack C.R., Albert M.S., Knopman D.S., McKhann G.M., Sperling R.A., Carrillo M.C., Thies B., Phelps C.H. Introduction to the recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. - DOI - PMC - PubMed
-
- Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Kaye J., Montine T.J., et al. Toward defining the preclinical stages of alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

