Development and validation of a physiology-based model for the prediction of pharmacokinetics/toxicokinetics in rabbits
- PMID: 29561908
- PMCID: PMC5862475
- DOI: 10.1371/journal.pone.0194294
Development and validation of a physiology-based model for the prediction of pharmacokinetics/toxicokinetics in rabbits
Abstract
The environmental fates of pharmaceuticals and the effects of crop protection products on non-target species are subjects that are undergoing intense review. Since measuring the concentrations and effects of xenobiotics on all affected species under all conceivable scenarios is not feasible, standard laboratory animals such as rabbits are tested, and the observed adverse effects are translated to focal species for environmental risk assessments. In that respect, mathematical modelling is becoming increasingly important for evaluating the consequences of pesticides in untested scenarios. In particular, physiologically based pharmacokinetic/toxicokinetic (PBPK/TK) modelling is a well-established methodology used to predict tissue concentrations based on the absorption, distribution, metabolism and excretion of drugs and toxicants. In the present work, a rabbit PBPK/TK model is developed and evaluated with data available from the literature. The model predictions include scenarios of both intravenous (i.v.) and oral (p.o.) administration of small and large compounds. The presented rabbit PBPK/TK model predicts the pharmacokinetics (Cmax, AUC) of the tested compounds with an average 1.7-fold error. This result indicates a good predictive capacity of the model, which enables its use for risk assessment modelling and simulations.
Conflict of interest statement
Figures
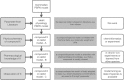
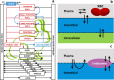
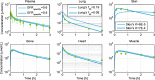
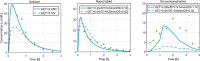

References
-
- Russell WMS, Burch RL. The principles of humane experimental technique. The principles of humane experimental technique. 1959.
-
- Hartung T. Food for thought… on animal tests. ALTEX. 2008;25(1):3–16. . - PubMed
-
- Leist M, Hartung T, Nicotera P. The dawning of a new age of toxicology. ALTEX. 2008;25(2):103–14. . - PubMed
-
- Scholz S, Sela E, Blaha L, Braunbeck T, Galay-Burgos M, Garcia-Franco M, et al. A European perspective on alternatives to animal testing for environmental hazard identification and risk assessment. Regul Toxicol Pharmacol. 2013;67(3):506–30. doi: 10.1016/j.yrtph.2013.10.003 . - DOI - PubMed
-
- Lilienblum W, Dekant W, Foth H, Gebel T, Hengstler JG, Kahl R, et al. Alternative methods to safety studies in experimental animals: role in the risk assessment of chemicals under the new European Chemicals Legislation (REACH). Arch Toxicol. 2008;82(4):211–36. doi: 10.1007/s00204-008-0279-9 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

