Moving magnetoencephalography towards real-world applications with a wearable system
- PMID: 29562238
- PMCID: PMC6063354
- DOI: 10.1038/nature26147
Moving magnetoencephalography towards real-world applications with a wearable system
Abstract
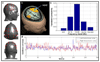
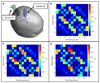
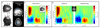
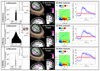
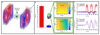
Imaging human brain function with techniques such as magnetoencephalography typically requires a subject to perform tasks while their head remains still within a restrictive scanner. This artificial environment makes the technique inaccessible to many people, and limits the experimental questions that can be addressed. For example, it has been difficult to apply neuroimaging to investigation of the neural substrates of cognitive development in babies and children, or to study processes in adults that require unconstrained head movement (such as spatial navigation). Here we describe a magnetoencephalography system that can be worn like a helmet, allowing free and natural movement during scanning. This is possible owing to the integration of quantum sensors, which do not rely on superconducting technology, with a system for nulling background magnetic fields. We demonstrate human electrophysiological measurement at millisecond resolution while subjects make natural movements, including head nodding, stretching, drinking and playing a ball game. Our results compare well to those of the current state-of-the-art, even when subjects make large head movements. The system opens up new possibilities for scanning any subject or patient group, with myriad applications such as characterization of the neurodevelopmental connectome, imaging subjects moving naturally in a virtual environment and investigating the pathophysiology of movement disorders.
Conflict of interest statement
The authors declare competing financial interests: co-author Vishal Shah is founding director of QuSpin – the commercial entity selling OPM magnetometers. QuSpin built the sensors used here and advised on the system design and operation, but played no part in the subsequent measurements or data analysis. This work was funded by a Wellcome award which involves a collaboration agreement with QuSpin.
Figures




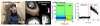
Similar articles
-
Precision magnetic field modelling and control for wearable magnetoencephalography.Neuroimage. 2021 Nov 1;241:118401. doi: 10.1016/j.neuroimage.2021.118401. Epub 2021 Jul 15. Neuroimage. 2021. PMID: 34273527 Free PMC article.
-
A bi-planar coil system for nulling background magnetic fields in scalp mounted magnetoencephalography.Neuroimage. 2018 Nov 1;181:760-774. doi: 10.1016/j.neuroimage.2018.07.028. Epub 2018 Jul 19. Neuroimage. 2018. PMID: 30031934 Free PMC article.
-
Wearable neuroimaging: Combining and contrasting magnetoencephalography and electroencephalography.Neuroimage. 2019 Nov 1;201:116099. doi: 10.1016/j.neuroimage.2019.116099. Epub 2019 Aug 14. Neuroimage. 2019. PMID: 31419612 Free PMC article.
-
Magnetoencephalography with optically pumped magnetometers (OPM-MEG): the next generation of functional neuroimaging.Trends Neurosci. 2022 Aug;45(8):621-634. doi: 10.1016/j.tins.2022.05.008. Epub 2022 Jun 30. Trends Neurosci. 2022. PMID: 35779970 Free PMC article. Review.
-
Multimodal fusion of magnetoencephalography and photoacoustic imaging based on optical pump: Trends for wearable and noninvasive Brain-Computer interface.Biosens Bioelectron. 2025 Jun 15;278:117321. doi: 10.1016/j.bios.2025.117321. Epub 2025 Feb 26. Biosens Bioelectron. 2025. PMID: 40049046 Review.
Cited by
-
Scalar Magnetometry Below 100 fT/Hz1/2 in a Microfabricated Cell.IEEE Sens J. 2020 Nov;20(21):12684-12690. doi: 10.1109/jsen.2020.3002193. Epub 2020 Jun 15. IEEE Sens J. 2020. PMID: 36275194 Free PMC article.
-
Mapping Interictal activity in epilepsy using a hidden Markov model: A magnetoencephalography study.Hum Brain Mapp. 2023 Jan;44(1):66-81. doi: 10.1002/hbm.26118. Epub 2022 Oct 19. Hum Brain Mapp. 2023. PMID: 36259549 Free PMC article.
-
Magnetic Source Imaging Using a Pulsed Optically Pumped Magnetometer Array.IEEE Trans Instrum Meas. 2019 Feb;68(2):493-501. doi: 10.1109/TIM.2018.2851458. Epub 2018 Jul 23. IEEE Trans Instrum Meas. 2019. PMID: 31777404 Free PMC article.
-
Brain Functional Connectivity Through Phase Coupling of Neuronal Oscillations: A Perspective From Magnetoencephalography.Front Neurosci. 2019 Sep 12;13:964. doi: 10.3389/fnins.2019.00964. eCollection 2019. Front Neurosci. 2019. PMID: 31572116 Free PMC article. Review.
-
Magnetocardiography on an isolated animal heart with a room-temperature optically pumped magnetometer.Sci Rep. 2018 Nov 1;8(1):16218. doi: 10.1038/s41598-018-34535-z. Sci Rep. 2018. PMID: 30385784 Free PMC article.
References
-
- Cohen D. Magnetoencephalography: Detection of the brains electrical activity with a superconducting magnetometer. Science. 1972;5:664–666. - PubMed
-
- Kominis IK, Kornack TW, Allred JC, Romalis MV. A subfemtotesla multichannel atomic magnetometer. Nature. 2003;422:596–599. - PubMed
-
- Hamalainen MS, Hari R, Ilmoniemi RJ, Knuutila J, Lounasma OV. Magnetoencephalography: Theory, instrumentation, and applications to non-invasive studies of the working human brain. Reviews of Modern Physics. 1993;65:413–497.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

