Revolution of Alzheimer Precision Neurology. Passageway of Systems Biology and Neurophysiology
- PMID: 29562524
- PMCID: PMC6008221
- DOI: 10.3233/JAD-179932
Revolution of Alzheimer Precision Neurology. Passageway of Systems Biology and Neurophysiology
Abstract
The Precision Neurology development process implements systems theory with system biology and neurophysiology in a parallel, bidirectional research path: a combined hypothesis-driven investigation of systems dysfunction within distinct molecular, cellular, and large-scale neural network systems in both animal models as well as through tests for the usefulness of these candidate dynamic systems biomarkers in different diseases and subgroups at different stages of pathophysiological progression. This translational research path is paralleled by an "omics"-based, hypothesis-free, exploratory research pathway, which will collect multimodal data from progressing asymptomatic, preclinical, and clinical neurodegenerative disease (ND) populations, within the wide continuous biological and clinical spectrum of ND, applying high-throughput and high-content technologies combined with powerful computational and statistical modeling tools, aimed at identifying novel dysfunctional systems and predictive marker signatures associated with ND. The goals are to identify common biological denominators or differentiating classifiers across the continuum of ND during detectable stages of pathophysiological progression, characterize systems-based intermediate endophenotypes, validate multi-modal novel diagnostic systems biomarkers, and advance clinical intervention trial designs by utilizing systems-based intermediate endophenotypes and candidate surrogate markers. Achieving these goals is key to the ultimate development of early and effective individualized treatment of ND, such as Alzheimer's disease. The Alzheimer Precision Medicine Initiative (APMI) and cohort program (APMI-CP), as well as the Paris based core of the Sorbonne University Clinical Research Group "Alzheimer Precision Medicine" (GRC-APM) were recently launched to facilitate the passageway from conventional clinical diagnostic and drug development toward breakthrough innovation based on the investigation of the comprehensive biological nature of aging individuals. The APMI movement is gaining momentum to systematically apply both systems neurophysiology and systems biology in exploratory translational neuroscience research on ND.
Keywords: Alzheimer’s disease; biomarkers; integrative disease modeling; pathophysiology; precision medicine; precision neurology; systems biology; systems neurophysiology; systems pharmacology; systems theory.
Figures



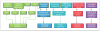

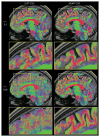


References
-
- Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. An analysis of prevalence, incidence, cost & trends. Alzheimer’s Disease International; London: 2015. World Alzheimer Report 2015. The global impact of dementia. Available at: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf.
-
- Baldacci F, Lista S, Cavedo E, Bonuccelli U, Hampel H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Rev Proteomics. 2017;14:285–299. - PubMed
-
- Baldacci F, Toschi N, Lista S, Zetterberg H, Blennow K, Kilimann I, Teipel S, Cavedo E, dos Santos AM, Epelbaum S, Lamari F, Dubois B, Floris R, Garaci F, Bonuccelli U, Hampel H. Two-level diagnostic classification using cerebrospinal fluid YKL-40 in Alzheimer’s disease. Alzheimer’s & Dementia. 2017;13:993–1003. - PubMed
-
- Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, Hölttä M, Rosén C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. The Lancet Neurology. 2016;15:673–684. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

