An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective
- PMID: 29562589
- PMCID: PMC5877750
- DOI: 10.3390/ijms19030889
An Interplay between Senescence, Apoptosis and Autophagy in Glioblastoma Multiforme-Role in Pathogenesis and Therapeutic Perspective
Abstract
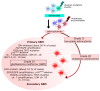
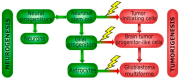
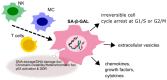
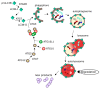
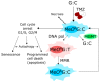
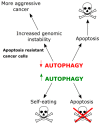
Autophagy, cellular senescence, programmed cell death and necrosis are key responses of a cell facing a stress. These effects are partly interconnected, but regulation of their mutual interactions is not completely clear. That regulation seems to be especially important in cancer cells, which have their own program of development and demand more nutrition and energy than normal cells. Glioblastoma multiforme (GBM) belongs to the most aggressive and most difficult to cure cancers, so studies on its pathogenesis and new therapeutic strategies are justified. Using an animal model, it was shown that autophagy is required for GBM development. Temozolomide (TMZ) is the key drug in GBM chemotherapy and it was reported to induce senescence, autophagy and apoptosis in GBM. In some GBM cells, TMZ induces small toxicity despite its significant concentration and GBM cells can be intrinsically resistant to apoptosis. Resveratrol, a natural compound, was shown to potentiate anticancer effect of TMZ in GBM cells through the abrogation G2-arrest and mitotic catastrophe resulting in senescence of GBM cells. Autophagy is the key player in TMZ resistance in GBM. TMZ can induce apoptosis due to selective inhibition of autophagy, in which autophagic vehicles accumulate as their fusion with lysosomes is blocked. Modulation of autophagic action of TMZ with autophagy inhibitors can result in opposite outcomes, depending on the step targeted in autophagic flux. Studies on relationships between senescence, autophagy and apoptosis can open new therapeutic perspectives in GBM.
Keywords: DNA damage response; apoptosis; autophagy; glioblastoma; senescence; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bourgeais J., Ishac N., Medrzycki M., Brachet-Botineau M., Desbourdes L., Gouilleux-Gruart V., Pecnard E., Rouleux-Bonnin F., Gyan E., Domenech J., et al. Oncogenic STAT5 signaling promotes oxidative stress in chronic myeloid leukemia cells by repressing antioxidant defenses. Oncotarget. 2017;8:41876–41889. doi: 10.18632/oncotarget.11480. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

