Optical Aggregation of Gold Nanoparticles for SERS Detection of Proteins and Toxins in Liquid Environment: Towards Ultrasensitive and Selective Detection
- PMID: 29562606
- PMCID: PMC5873019
- DOI: 10.3390/ma11030440
Optical Aggregation of Gold Nanoparticles for SERS Detection of Proteins and Toxins in Liquid Environment: Towards Ultrasensitive and Selective Detection
Abstract
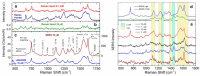
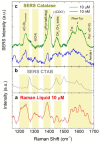
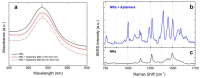
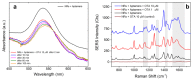
Optical forces are used to aggregate plasmonic nanoparticles and create SERS-active hot spots in liquid. When biomolecules are added to the nanoparticles, high sensitivity SERS detection can be accomplished. Here, we pursue studies on Bovine Serum Albumin (BSA) detection, investigating the BSA-nanorod aggregations in a range from 100 µM to 50 nM by combining light scattering, plasmon resonance and SERS, and correlating the SERS signal with the concentration. Experimental data are fitted with a simple model describing the optical aggregation process. We show that BSA-nanorod complexes can be optically printed on non-functionalized glass surfaces, designing custom patterns stable with time. Furthermore, we demonstrate that this methodology can be used to detect catalase and hemoglobin, two Raman resonant biomolecules, at concentrations of 10 nM and 1 pM, respectively, i.e., well beyond the limit of detection of BSA. Finally, we show that nanorods functionalized with specific aptamers can be used to capture and detect Ochratoxin A, a fungal toxin found in food commodities and wine. This experiment represents the first step towards the addition of molecular specificity to this novel biosensor strategy.
Keywords: SERS; aptamers; biosensor; colloids; gold nanoparticles; hemeprotein; optical forces; optical patterning; optical tweezers; toxins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
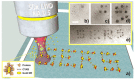
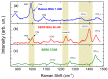

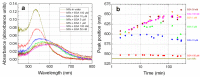
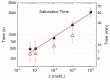


Similar articles
-
SERS detection of Biomolecules at Physiological pH via aggregation of Gold Nanorods mediated by Optical Forces and Plasmonic Heating.Sci Rep. 2016 Jun 1;6:26952. doi: 10.1038/srep26952. Sci Rep. 2016. PMID: 27246267 Free PMC article.
-
Combining 3-D plasmonic gold nanorod arrays with colloidal nanoparticles as a versatile concept for reliable, sensitive, and selective molecular detection by SERS.Phys Chem Chem Phys. 2014 Mar 28;16(12):5563-70. doi: 10.1039/c3cp55087g. Epub 2014 Feb 10. Phys Chem Chem Phys. 2014. PMID: 24514029
-
Ultrasensitive and selective homogeneous sandwich immunoassay detection by Surface Enhanced Raman Scattering (SERS).Analyst. 2012 Oct 21;137(20):4834-40. doi: 10.1039/c2an35471c. Analyst. 2012. PMID: 22943047
-
SERS detection of biomolecules using lithographed nanoparticles towards a reproducible SERS biosensor.Nanotechnology. 2010 Nov 26;21(47):475501. doi: 10.1088/0957-4484/21/47/475501. Epub 2010 Oct 29. Nanotechnology. 2010. PMID: 21030778
-
Surface-enhanced Raman scattering: realization of localized surface plasmon resonance using unique substrates and methods.Anal Bioanal Chem. 2009 Aug;394(7):1747-60. doi: 10.1007/s00216-009-2762-4. Epub 2009 Apr 22. Anal Bioanal Chem. 2009. PMID: 19384546 Review.
Cited by
-
Biosensing Using SERS Active Gold Nanostructures.Nanomaterials (Basel). 2021 Oct 12;11(10):2679. doi: 10.3390/nano11102679. Nanomaterials (Basel). 2021. PMID: 34685120 Free PMC article. Review.
-
Facile In Situ Photochemical Synthesis of Silver Nanoaggregates for Surface-Enhanced Raman Scattering Applications.Nanomaterials (Basel). 2020 Apr 5;10(4):685. doi: 10.3390/nano10040685. Nanomaterials (Basel). 2020. PMID: 32260572 Free PMC article.
-
A Review on Surface-Enhanced Raman Scattering.Biosensors (Basel). 2019 Apr 17;9(2):57. doi: 10.3390/bios9020057. Biosensors (Basel). 2019. PMID: 30999661 Free PMC article. Review.
-
Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy.Nanomaterials (Basel). 2018 Sep 15;8(9):730. doi: 10.3390/nano8090730. Nanomaterials (Basel). 2018. PMID: 30223597 Free PMC article. Review.
-
Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions.Nanomaterials (Basel). 2019 Nov 16;9(11):1630. doi: 10.3390/nano9111630. Nanomaterials (Basel). 2019. PMID: 31744124 Free PMC article.
References
-
- Le Ru E., Etchegoin P.G. Principles of Surface Enhanced Raman Spectroscopy. Elsevier; Amsterdam, The Netherlands: 2009.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

