Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery
- PMID: 29562677
- PMCID: PMC6017474
- DOI: 10.3390/molecules23030687
Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery
Abstract
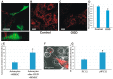
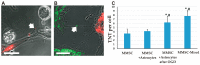
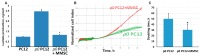
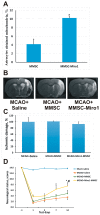
A recently discovered key role of reactive oxygen species (ROS) in mitochondrial traffic has opened a wide alley for studying the interactions between cells, including stem cells. Since its discovery in 2006, intercellular mitochondria transport has been intensively studied in different cellular models as a basis for cell therapy, since the potential of replacing malfunctioning organelles appears to be very promising. In this study, we explored the transfer of mitochondria from multipotent mesenchymal stem cells (MMSC) to neural cells and analyzed its efficacy under normal conditions and upon induction of mitochondrial damage. We found that mitochondria were transferred from the MMSC to astrocytes in a more efficient manner when the astrocytes were exposed to ischemic damage associated with elevated ROS levels. Such transport of mitochondria restored the bioenergetics of the recipient cells and stimulated their proliferation. The introduction of MMSC with overexpressed Miro1 in animals that had undergone an experimental stroke led to significantly improved recovery of neurological functions. Our data suggest that mitochondrial impairment in differentiated cells can be compensated by receiving healthy mitochondria from MMSC. We demonstrate a key role of Miro1, which promotes the mitochondrial transfer from MMSC and suggest that the genetic modification of stem cells can improve the therapies for the injured brain.
Keywords: astrocyte; ischemia; mitochondria; stroke; tunneling nanotubes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Shin B., Cowan D.B., Emani S.M., Del Nido P.J., McCully J.D. Mitochondrial transplantation in myocardial ischemia and reperfusion injury. Adv. Exp. Med. Biol. 2017;982:595–619. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

