Beet Necrotic Yellow Vein Virus Noncoding RNA Production Depends on a 5'→3' Xrn Exoribonuclease Activity
- PMID: 29562720
- PMCID: PMC5869530
- DOI: 10.3390/v10030137
Beet Necrotic Yellow Vein Virus Noncoding RNA Production Depends on a 5'→3' Xrn Exoribonuclease Activity
Abstract
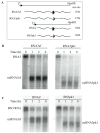
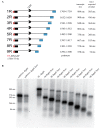
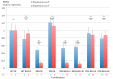
The RNA3 species of the beet necrotic yellow vein virus (BNYVV), a multipartite positive-stranded RNA phytovirus, contains the 'core' nucleotide sequence required for its systemic movement in Beta macrocarpa. Within this 'core' sequence resides a conserved "coremin" motif of 20 nucleotides that is absolutely essential for long-distance movement. RNA3 undergoes processing steps to yield a noncoding RNA3 (ncRNA3) possessing "coremin" at its 5' end, a mandatory element for ncRNA3 accumulation. Expression of wild-type (wt) or mutated RNA3 in Saccharomyces cerevisiae allows for the accumulation of ncRNA3 species. Screening of S.cerevisiae ribonuclease mutants identified the 5'-to-3' exoribonuclease Xrn1 as a key enzyme in RNA3 processing that was recapitulated both in vitro and in insect cell extracts. Xrn1 stalled on ncRNA3-containing RNA substrates in these decay assays in a similar fashion as the flavivirus Xrn1-resistant structure (sfRNA). Substitution of the BNYVV-RNA3 'core' sequence by the sfRNA sequence led to the accumulation of an ncRNA species in yeast in vitro but not in planta and no viral long distance occurred. Interestingly, XRN4 knockdown reduced BNYVV RNA accumulation suggesting a dual role for the ribonuclease in the viral cycle.
Keywords: BNYVV; VIGS; Viral noncoding RNA; exoribonuclease; flavivirus.
Conflict of interest statement
The authors declare no conflict of interest and state that Elodie Klein is an employee of SESV and has provided help with the RNA assays. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures






Similar articles
-
A Viral Noncoding RNA Complements a Weakened Viral RNA Silencing Suppressor and Promotes Efficient Systemic Host Infection.Viruses. 2016 Oct 4;8(10):272. doi: 10.3390/v8100272. Viruses. 2016. PMID: 27782046 Free PMC article.
-
Beet necrotic yellow vein virus subgenomic RNA3 is a cleavage product leading to stable non-coding RNA required for long-distance movement.J Gen Virol. 2012 May;93(Pt 5):1093-1102. doi: 10.1099/vir.0.039685-0. Epub 2012 Jan 18. J Gen Virol. 2012. PMID: 22258860
-
Detection and characterization of spontaneous internal deletion mutants of Beet Necrotic yellow vein virus RNA3 from systemic host Nicotiana benthamiana.Virol J. 2011 Jul 1;8:335. doi: 10.1186/1743-422X-8-335. Virol J. 2011. PMID: 21718549 Free PMC article.
-
Functional non-coding RNAs derived from the flavivirus 3' untranslated region.Virus Res. 2015 Aug 3;206:53-61. doi: 10.1016/j.virusres.2015.01.026. Epub 2015 Feb 7. Virus Res. 2015. PMID: 25660582 Review.
-
Noncoding RNAs of Plant Viruses and Viroids: Sponges of Host Translation and RNA Interference Machinery.Mol Plant Microbe Interact. 2016 Mar;29(3):156-64. doi: 10.1094/MPMI-10-15-0226-FI. Epub 2016 Feb 22. Mol Plant Microbe Interact. 2016. PMID: 26900786 Free PMC article. Review.
Cited by
-
Xrn1-resistant RNA motifs are disseminated throughout the RNA virome and are able to block scanning ribosomes.Sci Rep. 2023 Sep 25;13(1):15987. doi: 10.1038/s41598-023-43001-4. Sci Rep. 2023. PMID: 37749116 Free PMC article.
-
Screening bacterial effectors and human virus proteins in yeast to identify host factors driving tombusvirus RNA recombination: a role for autophagy and membrane phospholipid content.J Virol. 2025 Jun 17;99(6):e0166124. doi: 10.1128/jvi.01661-24. Epub 2025 May 27. J Virol. 2025. PMID: 40422074 Free PMC article.
-
Zika virus noncoding sfRNAs sequester multiple host-derived RNA-binding proteins and modulate mRNA decay and splicing during infection.J Biol Chem. 2019 Nov 1;294(44):16282-16296. doi: 10.1074/jbc.RA119.009129. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519749 Free PMC article.
-
Shared properties and singularities of exoribonuclease-resistant RNAs in viruses.Comput Struct Biotechnol J. 2021 Jul 26;19:4373-4380. doi: 10.1016/j.csbj.2021.07.024. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34471487 Free PMC article. Review.
-
The pseudoknot structure of a viral RNA reveals a conserved mechanism for programmed exoribonuclease resistance.bioRxiv [Preprint]. 2024 Dec 18:2024.12.17.628992. doi: 10.1101/2024.12.17.628992. bioRxiv. 2024. PMID: 39763890 Free PMC article. Preprint.
References
-
- Sokoloski K.J., Dickson A.M., Chaskey E.L., Garneau N.L., Wilusz C.J., Wilusz J. Sindbis virus usurps the cellular hur protein to stabilize its transcripts and promote productive infections in mammalian and mosquito cells. Cell Host Microbe. 2010;8:196–207. doi: 10.1016/j.chom.2010.07.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

