In Utero Transplantation of Placenta-Derived Mesenchymal Stromal Cells for Potential Fetal Treatment of Hemophilia A
- PMID: 29562772
- PMCID: PMC6434487
- DOI: 10.1177/0963689717728937
In Utero Transplantation of Placenta-Derived Mesenchymal Stromal Cells for Potential Fetal Treatment of Hemophilia A
Abstract
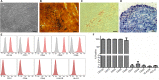
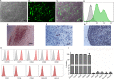
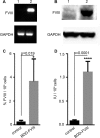
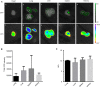
Hemophilia A (HA) is an X-linked recessive disorder caused by mutations in the factor VIII ( FVIII) gene leading to deficient blood coagulation. The current standard of care is frequent infusions of plasma-derived FVIII or recombinant B-domain-deleted FVIII (BDD-FVIII). While this treatment is effective, many patients eventually develop FVIII inhibitors that limit the effectiveness of the infused FVIII. As a monogenic disorder, HA is an ideal target for gene or cell-based therapy. Several studies have investigated allogeneic stem cell therapy targeting in utero or postnatal treatment of HA but have not been successful in completely correcting HA. Autologous in utero transplantation of mesenchymal stem cells is promising for treatment of HA due to the naive immune status of the fetal environment as well as its potential to prevent transplant rejection and long-term FVIII inhibitor formation. HA can be diagnosed by chorionic villus sampling performed during the first trimester (10 to 13 wk) of gestation. In this study, we used an established protocol and isolated placenta-derived mesenchymal stromal cells (PMSCs) from first trimester chorionic villus tissue and transduced them with lentiviral vector encoding the BDD-FVIII gene. We show that gene-modified PMSCs maintain their immunophenotype and multipotency, express, and secrete high levels of active FVIII. PMSCs were then transplanted at embryonic day 14.5 (E14.5) into wild-type fetuses from time-mated pregnant mice. Four days after birth, pups were checked for engraftment, and varying levels of expression of human green fluorescent protein were found in the organs tested. This study shows feasibility of the approach to obtain PMSCs from first trimester chorionic villus tissue, genetically modify them with the FVIII gene, and transplant them in utero for cell-mediated gene therapy of HA. Future studies will involve evaluation of long-term engraftment, phenotypic correction in HA mice, and prevention of FVIII inhibitor development by this approach.
Keywords: chorionic villus sampling; factor VIII; hemophilia A; in utero transplantation (IUT); placenta-derived mesenchymal stromal cells (PMSCs).
Conflict of interest statement
Figures
Similar articles
-
In Utero Cell Treatment of Hemophilia A Mice via Human Amniotic Fluid Mesenchymal Stromal Cell Engraftment.Int J Mol Sci. 2023 Nov 16;24(22):16411. doi: 10.3390/ijms242216411. Int J Mol Sci. 2023. PMID: 38003601 Free PMC article.
-
Potential long-term treatment of hemophilia A by neonatal co-transplantation of cord blood-derived endothelial colony-forming cells and placental mesenchymal stromal cells.Stem Cell Res Ther. 2019 Jan 22;10(1):34. doi: 10.1186/s13287-019-1138-8. Stem Cell Res Ther. 2019. PMID: 30670078 Free PMC article.
-
The mesenchymal stem cells derived from transgenic mice carrying human coagulation factor VIII can correct phenotype in hemophilia A mice.J Genet Genomics. 2013 Dec 20;40(12):617-28. doi: 10.1016/j.jgg.2013.11.002. Epub 2013 Nov 16. J Genet Genomics. 2013. PMID: 24377868
-
Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A.Curr Gene Ther. 2003 Feb;3(1):27-41. doi: 10.2174/1566523033347417. Curr Gene Ther. 2003. PMID: 12553533 Review.
-
Progress and challenges in the development of a cell-based therapy for hemophilia A.J Thromb Haemost. 2014 Dec;12(12):1954-65. doi: 10.1111/jth.12750. Epub 2014 Oct 31. J Thromb Haemost. 2014. PMID: 25297648 Free PMC article. Review.
Cited by
-
Acceptability of prenatal diagnosis and prenatal treatment of haemophilia using cell and gene therapies within US haemophilia community.Haemophilia. 2023 Jul;29(4):1024-1031. doi: 10.1111/hae.14805. Epub 2023 May 25. Haemophilia. 2023. PMID: 37228173 Free PMC article.
-
In Utero Cell Treatment of Hemophilia A Mice via Human Amniotic Fluid Mesenchymal Stromal Cell Engraftment.Int J Mol Sci. 2023 Nov 16;24(22):16411. doi: 10.3390/ijms242216411. Int J Mol Sci. 2023. PMID: 38003601 Free PMC article.
-
Restoration of FVIII Function and Phenotypic Rescue in Hemophilia A Mice by Transplantation of MSCs Derived From F8-Modified iPSCs.Front Cell Dev Biol. 2021 Feb 11;9:630353. doi: 10.3389/fcell.2021.630353. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33644070 Free PMC article.
-
In utero delivery of mRNA to the heart, diaphragm and muscle with lipid nanoparticles.Bioact Mater. 2023 Feb 17;25:387-398. doi: 10.1016/j.bioactmat.2023.02.011. eCollection 2023 Jul. Bioact Mater. 2023. PMID: 36844366 Free PMC article.
-
Stem Cell-Based Therapeutic Approaches in Genetic Diseases.Adv Exp Med Biol. 2023;1436:19-53. doi: 10.1007/5584_2023_761. Adv Exp Med Biol. 2023. PMID: 36735185
References
-
- Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet. 2003;361(9371):1801–1809. - PubMed
-
- Kulkarni R, Soucie JM, Lusher J, Presley R, Shapiro A, Gill J, Manco-Johnson M, Koerper M, Mathew P, Abshire T, et al. Sites of initial bleeding episodes, mode of delivery and age of diagnosis in babies with haemophilia diagnosed before the age of 2 years: a report from The Centers for Disease Control and Prevention’s (CDC) Universal Data Collection (UDC) project. Haemophilia. 2009;15(6):1281–1290. - PubMed
-
- Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco- Johnson ML, Funk S, Jacobson L, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. - PubMed
-
- Chalmers EA, Brown SA, Keeling D, Liesner R, Richards M, Stirling D, Thomas A, Vidler V, Williams MD, Young D, et al. Early factor VIII exposure and subsequent inhibitor development in children with severe haemophilia A. Haemophilia. 2007;13(2):149–155. - PubMed
-
- Gouw SC, van der Bom JG, Ljung R, Escuriola C, Cid AR, Claeyssens-Donadel S, van Geet C, Kenet G, Makipernaa A, Molinari AC, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368(3):231–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

