High-Dose Deferoxamine Treatment Disrupts Intracellular Iron Homeostasis, Reduces Growth, and Induces Apoptosis in Metastatic and Nonmetastatic Breast Cancer Cell Lines
- PMID: 29562821
- PMCID: PMC5865460
- DOI: 10.1177/1533033818764470
High-Dose Deferoxamine Treatment Disrupts Intracellular Iron Homeostasis, Reduces Growth, and Induces Apoptosis in Metastatic and Nonmetastatic Breast Cancer Cell Lines
Abstract
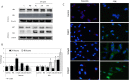
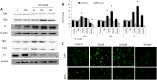
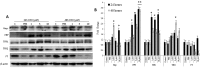
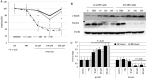
Mounting evidence suggest that iron overload enhances cancer growth and metastasis; hence, iron chelation is being increasingly used as part of the treatment regimen in patients with cancer. Now whether iron chelation depletes intracellular iron and/or disrupts intracellular iron homeostasis is yet to be fully addressed. MCF-7 and MDA-MB-231 breast cancer cells treated with increasing concentrations of the iron chelator deferoxamine were assessed for intracellular iron status, the expression of key proteins involved in iron metabolism, cell viability, growth potential, and apoptosis at different time points following treatment. Treatment with deferoxamine at 1, 5, or 10 μM for 24 or 48 hours, while not leading to significant changes in intracellular labile iron content, upregulated the expression of hepcidin, ferroportin, and transferrin receptors 1 and 2. In contrast, deferoxamine at 30, 100, or 300 μM for 24 hours induced a significant decrease in intracellular labile iron, which was associated with increased expression of hepcidin, ferritin, and transferrin receptors 1 and 2. At 48 hours, there was an increase in intracellular labile iron, which was associated with a significant reduction in hepcidin and ferritin expression and a significant increase in ferroportin expression. Although low-dose deferoxamine treatment resulted in a low to moderate decrease in MCF-7 cell growth, high-dose treatment resulted in a significant and precipitous decrease in cell viability and growth, which was associated with increased expression of phosphorylated Histone 2A family member X and near absence of survivin. High-dose deferoxamine treatment also resulted in a very pronounced reduction in wound healing and growth in MDA-MB-231 cells. These findings suggest that high-dose deferoxamine treatment disrupts intracellular iron homeostasis, reduces cell viability and growth, and enhances apoptosis in breast cancer cells. This is further evidence to the potential utility of iron chelation as an adjunctive therapy in iron-overloaded cancers.
Keywords: MCF-7; apoptosis; deferoxamine; ferritin; ferroportin; hepcidin; iron chelation.
Conflict of interest statement
Figures



References
-
- Anderson GJ, Wang F. Essential but toxic: controlling the flux of iron in the body. Clin Exp Pharmacol Physiol. 2012;39(8):719–724. - PubMed
-
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. - PubMed
-
- Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;3(9):1096–1101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

