Nano-Pulsed Laser Therapy Is Neuroprotective in a Rat Model of Blast-Induced Neurotrauma
- PMID: 29562823
- PMCID: PMC5998828
- DOI: 10.1089/neu.2017.5249
Nano-Pulsed Laser Therapy Is Neuroprotective in a Rat Model of Blast-Induced Neurotrauma
Abstract
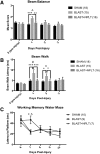
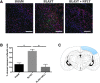
We have developed a novel, non-invasive nano-pulsed laser therapy (NPLT) system that combines the benefits of near-infrared laser light (808 nm) and ultrasound (optoacoustic) waves, which are generated with each short laser pulse within the tissue. We tested NPLT in a rat model of blast-induced neurotrauma (BINT) to determine whether transcranial application of NPLT provides neuroprotective effects. The laser pulses were applied on the intact rat head 1 h after injury using a specially developed fiber-optic system. Vestibulomotor function was assessed on post-injury days (PIDs) 1-3 on the beam balance and beam walking tasks. Cognitive function was assessed on PIDs 6-10 using a working memory Morris water maze (MWM) test. BDNF and caspase-3 messenger RNA (mRNA) expression was measured by quantitative real-time PCR (qRT-PCR) in laser-captured cortical neurons. Microglia activation and neuronal injury were assessed in brain sections by immunofluorescence using specific antibodies against CD68 and active caspase-3, respectively. In the vestibulomotor and cognitive (MWM) tests, NPLT-treated animals performed significantly better than the untreated blast group and similarly to sham animals. NPLT upregulated mRNA encoding BDNF and downregulated the pro-apoptotic protein caspase-3 in cortical neurons. Immunofluorescence demonstrated that NPLT inhibited microglia activation and reduced the number of cortical neurons expressing activated caspase-3. NPLT also increased expression of BDNF in the hippocampus and the number of proliferating progenitor cells in the dentate gyrus. Our data demonstrate a neuroprotective effect of NPLT and prompt further studies aimed to develop NPLT as a therapeutic intervention after traumatic brain injury (TBI).
Keywords: blast injury; near-infrared light; neuroprotection; non-invasive transcranial laser therapy; optoacoustics; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Figures





Similar articles
-
Non-Invasive Transcranial Nano-Pulsed Laser Therapy Ameliorates Cognitive Function and Prevents Aberrant Migration of Neural Progenitor Cells in the Hippocampus of Rats Subjected to Traumatic Brain Injury.J Neurotrauma. 2020 Apr 15;37(8):1108-1123. doi: 10.1089/neu.2019.6534. Epub 2020 Jan 31. J Neurotrauma. 2020. PMID: 31856661
-
Endoplasmic Reticulum Stress Modulation as a Target for Ameliorating Effects of Blast Induced Traumatic Brain Injury.J Neurotrauma. 2017 Sep;34(S1):S62-S70. doi: 10.1089/neu.2016.4680. Epub 2017 Feb 27. J Neurotrauma. 2017. PMID: 28077004 Free PMC article.
-
Effects of Mild Blast Traumatic Brain Injury on Cerebral Vascular, Histopathological, and Behavioral Outcomes in Rats.J Neurotrauma. 2018 Jan 15;35(2):375-392. doi: 10.1089/neu.2017.5256. Epub 2017 Dec 20. J Neurotrauma. 2018. PMID: 29160141 Free PMC article.
-
Combat blast related traumatic brain injury (TBI): Decade of recognition; promise of progress.Behav Brain Res. 2018 Mar 15;340:102-105. doi: 10.1016/j.bbr.2016.08.036. Epub 2016 Aug 20. Behav Brain Res. 2018. PMID: 27555540 Review.
-
Transcranial, Red/Near-Infrared Light-Emitting Diode Therapy to Improve Cognition in Chronic Traumatic Brain Injury.Photomed Laser Surg. 2016 Dec;34(12):610-626. doi: 10.1089/pho.2015.4037. Photomed Laser Surg. 2016. PMID: 28001756 Review.
Cited by
-
The Role of BDNF in Experimental and Clinical Traumatic Brain Injury.Int J Mol Sci. 2021 Mar 30;22(7):3582. doi: 10.3390/ijms22073582. Int J Mol Sci. 2021. PMID: 33808272 Free PMC article. Review.
-
The Effects of Unilateral Labyrinthectomy on Monoamine Neurotransmitters in the Medial Vestibular Nucleus of Rats.Biomolecules. 2023 Nov 10;13(11):1637. doi: 10.3390/biom13111637. Biomolecules. 2023. PMID: 38002319 Free PMC article.
-
Paths to Successful Translation of New Therapies for Severe Traumatic Brain Injury in the Golden Age of Traumatic Brain Injury Research: A Pittsburgh Vision.J Neurotrauma. 2020 Nov 15;37(22):2353-2371. doi: 10.1089/neu.2018.6203. Epub 2019 Feb 1. J Neurotrauma. 2020. PMID: 30520681 Free PMC article. Review.
-
Transcranial photobiomodulation for brain diseases: review of animal and human studies including mechanisms and emerging trends.Neurophotonics. 2024 Jan;11(1):010601. doi: 10.1117/1.NPh.11.1.010601. Epub 2024 Feb 5. Neurophotonics. 2024. PMID: 38317779 Free PMC article. Review.
-
Comprehensive Characterization of Cerebrovascular Dysfunction in Blast Traumatic Brain Injury Using Photoacoustic Microscopy.J Neurotrauma. 2019 May 15;36(10):1526-1534. doi: 10.1089/neu.2018.6062. Epub 2019 Jan 25. J Neurotrauma. 2019. PMID: 30501547 Free PMC article.
References
-
- Hyatt K., Davis L.L., and Barroso J. (2014). Chasing the care: soldiers experience following combat-related mild traumatic brain injury. Mil. Med. 179, 849–855 - PubMed
-
- Hoge C.W. (2008). Re: “Psychiatric diagnoses in historic and contemporary military cohorts: combat deployment and the healthy warrior effect.” Am. J. Epidemiol. 168, 1095–1096; author reply 1096–1098 - PubMed
-
- Wilk J.E., Thomas J.L., McGurk D.M., Riviere L.A., Castro C.A., and Hoge C.W. (2010). Mild traumatic brain injury (concussion) during combat: lack of association of blast mechanism with persistent postconcussive symptoms. J. Head Trauma Rehabil. 25, 9–14 - PubMed
-
- Stein M.B., Ursano R.J., Campbell-Sills L., Colpe L.J., Fullerton C.S., Heeringa S.G., Nock M.K., Sampson N.A., Schoenbaum M., Sun X., Jain S., and Kessler R.C. (2016). Prognostic indicators of persistent post-concussive symptoms after deployment-related mild traumatic brain injury: a prospective longitudinal study in U.S. Army soldiers. J. Neurotrauma 33, 2125–2132 - PMC - PubMed
-
- Boyle E., Cancelliere C., Hartvigsen J., Carroll L.J., Holm L.W., and Cassidy J.D. (2014). Systematic review of prognosis after mild traumatic brain injury in the military: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 95, S230–S237 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
