The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis - a secondary analysis of a large randomised controlled trial
- PMID: 29562917
- PMCID: PMC5863464
- DOI: 10.1186/s13054-018-2001-5
The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis - a secondary analysis of a large randomised controlled trial
Abstract
Background: This study assessed the ability of mid-regional proadrenomedullin (MR-proADM) in comparison to conventional biomarkers (procalcitonin (PCT), lactate, C-reactive protein) and clinical scores to identify disease severity in patients with sepsis.
Methods: This is a secondary analysis of a randomised controlled trial in patients with severe sepsis or septic shock across 33 German intensive care units. The association between biomarkers and clinical scores with mortality was assessed by Cox regression analysis, area under the receiver operating characteristic and Kaplan-Meier curves. Patients were stratified into three severity groups (low, intermediate, high) for all biomarkers and scores based on cutoffs with either a 90% sensitivity or specificity.
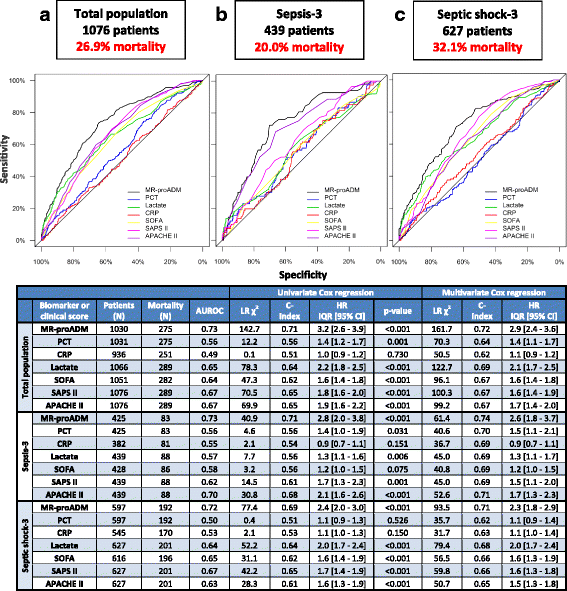
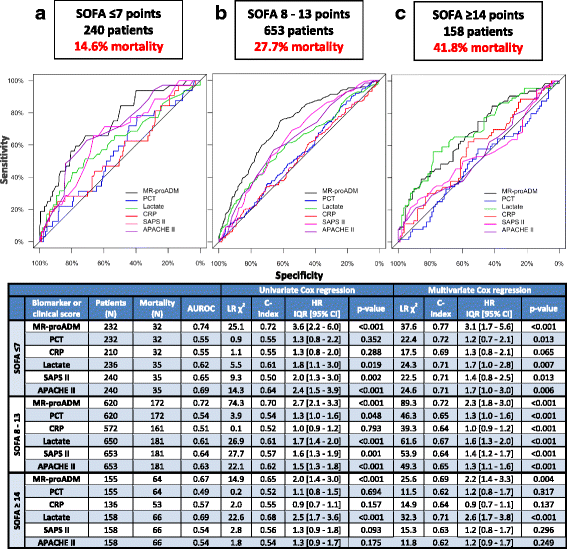
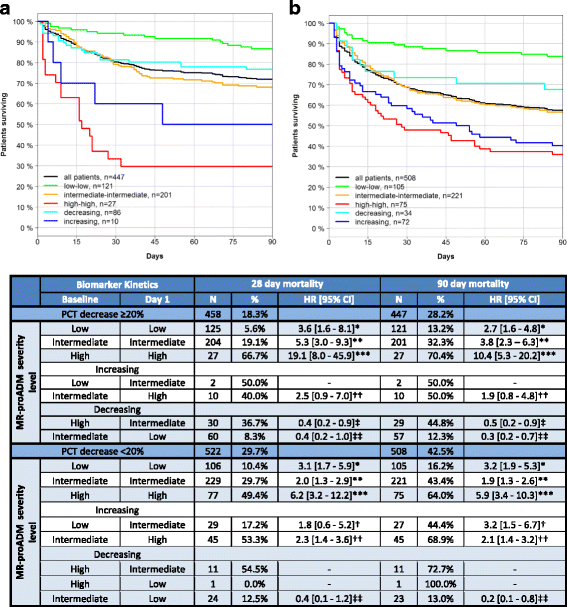
Results: 1089 patients with a 28-day mortality rate of 26.9% were analysed. According to the Sepsis-3 definition, 41.2% and 58.8% fulfilled the criteria for sepsis and septic shock, with respective mortality rates of 20.0% and 32.1%. MR-proADM had the strongest association with mortality across all Sepsis-1 and Sepsis-3 subgroups and could facilitate a more accurate classification of low (e.g. MR-proADM vs. SOFA: N = 265 vs. 232; 9.8% vs. 13.8% mortality) and high (e.g. MR-proADM vs. SOFA: N = 161 vs. 155; 55.9% vs. 41.3% mortality) disease severity. Patients with decreasing PCT concentrations of either ≥ 20% (baseline to day 1) or ≥ 50% (baseline to day 4) but continuously high MR-proADM concentrations had a significantly increased mortality risk (HR (95% CI): 19.1 (8.0-45.9) and 43.1 (10.1-184.0)).
Conclusions: MR-proADM identifies disease severity and treatment response more accurately than established biomarkers and scores, adding additional information to facilitate rapid clinical decision-making and improve personalised sepsis treatment.
Keywords: Biomarkers; MR-proADM; Mortality; SOFA; Sepsis; Septic shock.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol of the SISPCT trial was approved by the ethics board of Jena University Hospital (Internal File No. 2242-03/08). Written informed consent was obtained from all patients or their legal representatives.
Consent for publication
No individual participant data are reported that would require consent from the participant (or legal parent or guardian for children) to publish.
Competing interests
All authors have provided information on potential conflicts of interests directly or indirectly related to the work submitted in the journal’s disclosure forms. FB reported receiving lecture honoraria from biosyn, Gilead, and CSL Behring and public funding for the SISPCT trial to his department by the German Federal Ministry of Education and Research, and unrestricted research grants for the SISPCT trial from biosyn and Thermo Fisher Scientific. DCW is an employee of BRAHMS GmbH. KR reported receiving personal fees from Adrenomed and being a shareholder of InflaRx Jena. SK reported receiving lecture fees from Astellas, Basilea, biotest, CSL Behring, CytoSorbents, Fresenius, Gilead, MSD, Pfizer and Thermo Fisher Scientific and being a member of advisory boards for Astellas, Fresenius, Gilead, MSD, Novartis and Pfizer. AN reported receiving lecture honoraria from Thermo Fisher Scientific. All other authors declared that they have no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Comment in
-
The challenge of removal of sepsis markers by continuous hemofiltration.Crit Care. 2019 May 15;23(1):173. doi: 10.1186/s13054-019-2464-z. Crit Care. 2019. PMID: 31092274 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

