miR-632 Promotes Laryngeal Carcinoma Cell Proliferation, Migration, and Invasion Through Negative Regulation of GSK3β
- PMID: 29562960
- PMCID: PMC7851529
- DOI: 10.3727/096504018X15213142076069
miR-632 Promotes Laryngeal Carcinoma Cell Proliferation, Migration, and Invasion Through Negative Regulation of GSK3β
Abstract
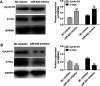
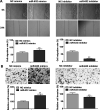
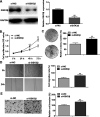
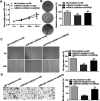
Laryngeal cancer, one of the most common head and neck malignancies, is an aggressive neoplasm. Increasing evidence has demonstrated that microRNAs (miRNAs) exert important roles in oncogenesis and progression of diverse types of human cancers. miR-632, a tumor-related miRNA, has been reported to be dysregulated and implicated in human malignancies; however, its biological role in laryngeal carcinoma remains to be elucidated. The present study aimed at exploring the role of miR-632 in laryngeal cancer and clarifying the potential molecular mechanisms involved. In the current study, miR-632 was found to be significantly upregulated both in laryngeal cancer tissues and laryngeal cancer cell lines. Functional studies demonstrated that miR-632 accelerated cell proliferation and colony formation, facilitated cell migration and invasion, and enhanced the expression of cell proliferation-associated proteins, cyclin D1 and c-myc. Notably, miR-632 could directly bind to the 3'-untranslated region (3'-UTR) of glycogen synthase kinase 3β (GSK3β) to suppress its expression in laryngeal cancer cells. Mechanical studies revealed that miR-632 promoted laryngeal cancer cell proliferation, migration, and invasion through negative modulation of GSK3β. Pearson's correlation analysis revealed that miR-632 expression was inversely correlated with GSK3β mRNA expression in laryngeal cancer tissues. Taken together, our findings suggest that miR-632 functions as an oncogene in laryngeal cancer and may be used as a novel therapeutic target for laryngeal cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Downregulation of miR-34a contributes to the proliferation and migration of laryngeal carcinoma cells by targeting cyclin D1.Oncol Rep. 2016 Jul;36(1):390-8. doi: 10.3892/or.2016.4823. Epub 2016 May 19. Oncol Rep. 2016. PMID: 27220728
-
Reciprocal regulation of miR-1205 and E2F1 modulates progression of laryngeal squamous cell carcinoma.Cell Death Dis. 2019 Dec 4;10(12):916. doi: 10.1038/s41419-019-2154-4. Cell Death Dis. 2019. PMID: 31801947 Free PMC article.
-
miR-205 promotes proliferation and invasion of laryngeal squamous cell carcinoma by suppressing CDK2AP1 expression.Biol Res. 2015 Oct 29;48:60. doi: 10.1186/s40659-015-0052-5. Biol Res. 2015. PMID: 26515287 Free PMC article.
-
The role of microRNAs expression in laryngeal cancer.Oncotarget. 2015 Sep 15;6(27):23297-305. doi: 10.18632/oncotarget.4195. Oncotarget. 2015. PMID: 26079642 Free PMC article. Review.
-
GSK3β as a novel promising target to overcome chemoresistance in pancreatic cancer.Drug Resist Updat. 2021 Sep;58:100779. doi: 10.1016/j.drup.2021.100779. Epub 2021 Aug 12. Drug Resist Updat. 2021. PMID: 34461526 Review.
Cited by
-
NLR, PLR, LMR and MWR as diagnostic and prognostic markers for laryngeal carcinoma.Am J Transl Res. 2022 May 15;14(5):3017-3027. eCollection 2022. Am J Transl Res. 2022. PMID: 35702077 Free PMC article.
-
miR-206 Inhibits Laryngeal Carcinoma Cell Multiplication, Migration, and Invasion.J Healthc Eng. 2021 Nov 26;2021:5614861. doi: 10.1155/2021/5614861. eCollection 2021. J Healthc Eng. 2021. Retraction in: J Healthc Eng. 2023 Jun 21;2023:9783934. doi: 10.1155/2023/9783934. PMID: 34868522 Free PMC article. Retracted.
-
Identification of core miRNA prognostic markers in patients with laryngeal cancer using bioinformatics analysis.Eur Arch Otorhinolaryngol. 2021 May;278(5):1613-1626. doi: 10.1007/s00405-020-06275-2. Epub 2020 Aug 12. Eur Arch Otorhinolaryngol. 2021. PMID: 32789639
-
miR-632 Induces DNAJB6 Inhibition Stimulating Endothelial-to-Mesenchymal Transition and Fibrosis in Marfan Syndrome Aortopathy.Int J Mol Sci. 2023 Oct 13;24(20):15133. doi: 10.3390/ijms242015133. Int J Mol Sci. 2023. PMID: 37894814 Free PMC article.
-
A frog peptide provides new strategies for the intervention against skin wound healing.Cell Mol Biol Lett. 2023 Jul 28;28(1):61. doi: 10.1186/s11658-023-00468-3. Cell Mol Biol Lett. 2023. PMID: 37501100 Free PMC article.
References
-
- Liu Y, Liu J, Wang L, Yang X, Liu X. MicroRNA195 inhibits cell proliferation, migration and invasion in laryngeal squamous cell carcinoma by targeting ROCK1. Mol Med Rep. 2017;16(5):7154–62. - PubMed
-
- Wu Q, Zhuang K, Li H. PAQR3 plays a suppressive role in laryngeal squamous cell carcinoma. Tumour Biol. 2016;37(1):561–5. - PubMed
-
- Wu CP, Zhou L, Gong HL, Du HD, Tian J, Sun S, Li JY. Establishment and characterization of a novel HPV-negative laryngeal squamous cell carcinoma cell line, FD-LSC-1, with missense and nonsense mutations of TP53 in the DNA-binding domain. Cancer Lett. 2014;342(1):92–103. - PubMed
-
- Langevin SM, Stone RA, Bunker CH, Lyons-Weiler MA, Laframboise WA, Kelly L, Seethala RR, Grandis JR, Sobol RW, Taioli E. MicroRNA-137 promoter methylation is associated with poorer overall survival in patients with squamous cell carcinoma of the head and neck. Cancer 2011;117(7):1454–62. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
