Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study
- PMID: 29563098
- PMCID: PMC5861502
- DOI: 10.1136/bmj.k872
Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study
Abstract
Objective: To assess whether the use of dipeptidyl peptidase-4 inhibitors is associated with the incidence of inflammatory bowel disease in patients with type 2 diabetes.
Design: Population based cohort study.
Setting: More than 700 general practices contributing data to the United Kingdom Clinical Practice Research Datalink.
Participants: A cohort of 141 170 patients, at least 18 years of age, starting antidiabetic drugs between 1 January 2007 and 31 December 2016, with follow-up until 30 June 2017.
Main outcome measures: Adjusted hazard ratios for incident inflammatory bowel disease associated with use of dipeptidyl peptidase-4 inhibitors overall, by cumulative duration of use, and by time since initiation, estimated using time dependent Cox proportional hazards models. Use of dipeptidyl peptidase-4 inhibitors was modelled as a time varying variable and compared with use of other antidiabetic drugs, with exposures lagged by six months to account for latency and diagnostic delays.
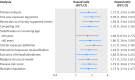
Results: During 552 413 person years of follow-up, 208 incident inflammatory bowel disease events occurred (crude incidence rate of 37.7 (95% confidence interval 32.7 to 43.1) per 100 000 person years). Overall, use of dipeptidyl peptidase-4 inhibitors was associated with an increased risk of inflammatory bowel disease (53.4 v 34.5 per 100 000 person years; hazard ratio 1.75, 95% confidence interval 1.22 to 2.49). Hazard ratios gradually increased with longer durations of use, reaching a peak after three to four years of use (hazard ratio 2.90, 1.31 to 6.41) and decreasing after more than four years of use (1.45, 0.44 to 4.76). A similar pattern was observed with time since starting dipeptidyl peptidase-4 inhibitors. These findings remained consistent in several sensitivity analyses.
Conclusions: In this first population based study, the use of dipeptidyl peptidase-4 inhibitors was associated with an increased risk of inflammatory bowel disease. Although these findings need to be replicated, physicians should be aware of this possible association.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: this study was funded by the Canadian Institutes of Health Research; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
IBD - a potential adverse effect of DDP4 inhibitors in T2DM.Nat Rev Endocrinol. 2018 Jun;14(6):323. doi: 10.1038/s41574-018-0007-3. Nat Rev Endocrinol. 2018. PMID: 29626203 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical