Chimeric Antigen Receptor T Cell-Mediated Neurotoxicity in Nonhuman Primates
- PMID: 29563103
- PMCID: PMC6058704
- DOI: 10.1158/2159-8290.CD-17-1368
Chimeric Antigen Receptor T Cell-Mediated Neurotoxicity in Nonhuman Primates
Abstract
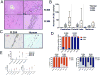
Chimeric antigen receptor (CAR) T-cell immunotherapy has revolutionized the treatment of refractory leukemias and lymphomas, but is associated with significant toxicities, namely cytokine release syndrome (CRS) and neurotoxicity. A major barrier to developing therapeutics to prevent CAR T cell-mediated neurotoxicity is the lack of clinically relevant models. Accordingly, we developed a rhesus macaque (RM) model of neurotoxicity via adoptive transfer of autologous CD20-specific CAR T cells. Following cyclophosphamide lymphodepletion, CD20 CAR T cells expand to 272 to 4,450 cells/μL after 7 to 8 days and elicit CRS and neurotoxicity. Toxicities are associated with elevated serum IL6, IL8, IL1RA, MIG, and I-TAC levels, and disproportionately high cerebrospinal fluid (CSF) IL6, IL2, GM-CSF, and VEGF levels. During neurotoxicity, both CD20 CAR and non-CAR T cells accumulate in the CSF and in the brain parenchyma. This RM model demonstrates that CAR T cell-mediated neurotoxicity is associated with proinflammatory CSF cytokines and a pan-T cell encephalitis.Significance: We provide the first immunologically relevant, nonhuman primate model of B cell-directed CAR T-cell therapy-mediated CRS and neurotoxicity. We demonstrate CAR and non-CAR T-cell infiltration in the CSF and in the brain during neurotoxicity resulting in pan-encephalitis, accompanied by increased levels of proinflammatory cytokines in the CSF. Cancer Discov; 8(6); 750-63. ©2018 AACR.This article is highlighted in the In This Issue feature, p. 663.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures
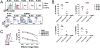



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

