Comparison of the Relative Potential for Epigenetic and Genetic Variation To Contribute to Trait Stability
- PMID: 29563187
- PMCID: PMC5940164
- DOI: 10.1534/g3.118.200127
Comparison of the Relative Potential for Epigenetic and Genetic Variation To Contribute to Trait Stability
Abstract
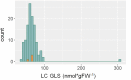
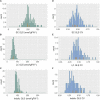
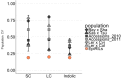
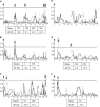
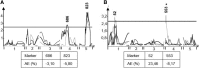
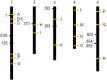
The theoretical ability of epigenetic variation to influence the heritable variation of complex traits is gaining traction in the study of adaptation. This theory posits that epigenetic marks can control adaptive phenotypes but the relative potential of epigenetic variation in comparison to genetic variation in these traits is not presently understood. To compare the potential of epigenetic and genetic variation in adaptive traits, we analyzed the influence of DNA methylation variation on the accumulation of chemical defense compounds glucosinolates from the order Brassicales. Several decades of work on glucosinolates has generated extensive knowledge about their synthesis, regulation, genetic variation and contribution to fitness establishing this pathway as a model pathway for complex adaptive traits. Using high-throughput phenotyping with a randomized block design of ddm1 derived Arabidopsis thaliana epigenetic Recombinant Inbred Lines, we measured the correlation between DNA methylation variation and mean glucosinolate variation and within line stochastic variation. Using this information, we identified epigenetic Quantitative Trait Loci that contained specific Differentially Methylated Regions associated with glucosinolate traits. This showed that variation in DNA methylation correlates both with levels and variance of glucosinolates and flowering time with trait-specific loci. By conducting a meta-analysis comparing the results to different genetically variable populations, we conclude that the influence of DNA methylation variation on these adaptive traits is much lower than the corresponding impact of standing genetic variation. As such, selective pressure on these traits should mainly affect standing genetic variation to lead to adaptation.
Keywords: DNA methylation; Local adaptation; epiQTL mapping; glucosinolates; trait stability.
Copyright © 2018 Aller et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
