Genetic mapping of species differences via in vitro crosses in mouse embryonic stem cells
- PMID: 29563231
- PMCID: PMC5889640
- DOI: 10.1073/pnas.1717474115
Genetic mapping of species differences via in vitro crosses in mouse embryonic stem cells
Abstract
Discovering the genetic changes underlying species differences is a central goal in evolutionary genetics. However, hybrid crosses between species in mammals often suffer from hybrid sterility, greatly complicating genetic mapping of trait variation across species. Here, we describe a simple, robust, and transgene-free technique to generate "in vitro crosses" in hybrid mouse embryonic stem (ES) cells by inducing random mitotic cross-overs with the drug ML216, which inhibits the DNA helicase Bloom syndrome (BLM). Starting with an interspecific F1 hybrid ES cell line between the Mus musculus laboratory mouse and Mus spretus (∼1.5 million years of divergence), we mapped the genetic basis of drug resistance to the antimetabolite tioguanine to a single region containing hypoxanthine-guanine phosphoribosyltransferase (Hprt) in as few as 21 d through "flow mapping" by coupling in vitro crosses with fluorescence-activated cell sorting (FACS). We also show how our platform can enable direct study of developmental variation by rederiving embryos with contribution from the recombinant ES cell lines. We demonstrate how in vitro crosses can overcome major bottlenecks in mouse complex trait genetics and address fundamental questions in evolutionary biology that are otherwise intractable through traditional breeding due to high cost, small litter sizes, and/or hybrid sterility. In doing so, we describe an experimental platform toward studying evolutionary systems biology in mouse and potentially in human and other mammals, including cross-species hybrids.
Keywords: QTL mapping; evolution; genetics; interspecific hybrids; mitotic recombination.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

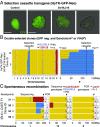
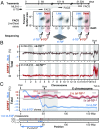
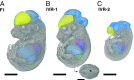
References
-
- Darwin C. On the Origin of Species by Means of Natural Selection. John Murray; London: 1859.
-
- Dejager L, Libert C, Montagutelli X. Thirty years of Mus spretus: A promising future. Trends Genet. 2009;25:234–241. - PubMed
-
- Mackay TFC, Stone EA, Ayroles JF. The genetics of quantitative traits: Challenges and prospects. Nat Rev Genet. 2009;10:565–577. - PubMed
-
- Allen Orr H. The genetics of species differences. Trends Ecol Evol. 2001;16:343–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

