Phosphorylation of MCAD selectively rescues PINK1 deficiencies in behavior and metabolism
- PMID: 29563254
- PMCID: PMC5935071
- DOI: 10.1091/mbc.E18-03-0155
Phosphorylation of MCAD selectively rescues PINK1 deficiencies in behavior and metabolism
Abstract
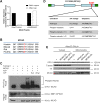
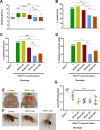
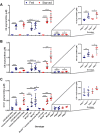
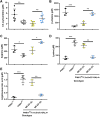
PTEN-induced putative kinase 1 (PINK1) is a mitochondria-targeted kinase whose mutations are a cause of Parkinson's disease. We set out to better understand PINK1's effects on mitochondrial proteins in vivo. Using an unbiased phosphoproteomic screen in Drosophila, we found that PINK1 mediates the phosphorylation of MCAD, a mitochondrial matrix protein critical to fatty acid metabolism. By mimicking phosphorylation of this protein in a PINK1 null background, we restored PINK1 null's climbing, flight, thorax, and wing deficiencies. Owing to MCAD's role in fatty acid metabolism, we examined the metabolic profile of PINK1 null flies, where we uncovered significant disruptions in both acylcarnitines and amino acids. Some of these disruptions were rescued by phosphorylation of MCAD, consistent with MCAD's rescue of PINK1 null's organismal phenotypes. Our work validates and extends the current knowledge of PINK1, identifies a novel function of MCAD, and illuminates the need for and effectiveness of metabolic profiling in models of neurodegenerative disease.
Figures
References
-
- Alves E, Henriques BJ, Rodrigues JV, Prudêncio P, Rocha H, Vilarinho L, Martinho RG, Gomes CM. (2012). Mutations at the flavin binding site of ETF: QO yield a MADD-like severe phenotype in Drosophila. Biochim Biophys Acta , 1284–1292. - PubMed
-
- Bian Y, Song C, Cheng K, Dong M, Wang F, Huang J, Sun D, Wang L, Ye M, Zou H. (2014). An enzyme assisted RP-RPLC approach for in-depth analysis of human liver phosphoproteome. J Proteomics , 253–262. - PubMed
-
- Brand AH, Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development , 401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

