Central Nervous System Inflammation and Infection during Early, Nonaccelerated Simian-Human Immunodeficiency Virus Infection in Rhesus Macaques
- PMID: 29563297
- PMCID: PMC5952152
- DOI: 10.1128/JVI.00222-18
Central Nervous System Inflammation and Infection during Early, Nonaccelerated Simian-Human Immunodeficiency Virus Infection in Rhesus Macaques
Abstract
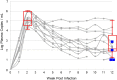
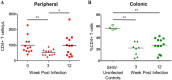
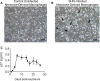
Studies utilizing highly pathogenic simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV) have largely focused on the immunopathology of the central nervous system (CNS) during end-stage neurological AIDS and SIV encephalitis. However, this may not model pathophysiology in earlier stages of infection. In this nonaccelerated SHIV model, plasma SHIV RNA levels and peripheral blood and colonic CD4+ T cell counts mirrored early human immunodeficiency virus (HIV) infection in humans. At 12 weeks postinfection, cerebrospinal fluid (CSF) detection of SHIV RNA and elevations in IP-10 and MCP-1 reflected a discrete neurovirologic process. Immunohistochemical staining revealed a diffuse, low-level CD3+ CD4- cellular infiltrate in the brain parenchyma without a concomitant increase in CD68/CD163+ monocytes, macrophages, and activated microglial cells. Rare SHIV-infected cells in the brain parenchyma and meninges were identified by RNAScope in situ hybridization. In the meninges, there was also a trend toward increased CD4+ infiltration in SHIV-infected animals but no differences in CD68/CD163+ cells between SHIV-infected and uninfected control animals. These data suggest that in a model that closely recapitulates human disease, CNS inflammation and SHIV in CSF are predominantly mediated by T cell-mediated processes during early infection in both brain parenchyma and meninges. Because SHIV expresses an HIV rather than SIV envelope, this model could inform studies to understand potential HIV cure strategies targeting the HIV envelope.IMPORTANCE Animal models of the neurologic effects of HIV are needed because brain pathology is difficult to assess in humans. Many current models focus on the effects of late-stage disease utilizing SIV. In the era of antiretroviral therapy, manifestations of late-stage HIV are less common. Furthermore, new interventions, such as monoclonal antibodies and therapeutic vaccinations, target HIV envelope. We therefore describe a new model of central nervous system involvement in rhesus macaques infected with SHIV expressing HIV envelope in earlier, less aggressive stages of disease. Here, we demonstrate that SHIV mimics the early clinical course in humans and that early neurologic inflammation is characterized by predominantly T cell-mediated inflammation accompanied by SHIV infection in the brain and meninges. This model can be utilized to assess the effect of novel therapies targeted to HIV envelope on reducing brain inflammation before end-stage disease.
Keywords: HIV; SHIV; early infection; neuropathology.
Copyright © 2018 Hsu et al.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

