TNF and granulocyte macrophage-colony stimulating factor interdependence mediates inflammation via CCL17
- PMID: 29563337
- PMCID: PMC5926922
- DOI: 10.1172/jci.insight.99249
TNF and granulocyte macrophage-colony stimulating factor interdependence mediates inflammation via CCL17
Abstract
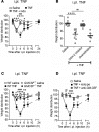
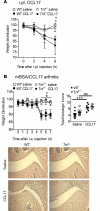
TNF and granulocyte macrophage-colony stimulating factor (GM-CSF) have proinflammatory activity and both contribute, for example, to rheumatoid arthritis pathogenesis. We previously identified a new GM-CSF→JMJD3 demethylase→interferon regulatory factor 4 (IRF4)→CCL17 pathway that is active in monocytes/macrophages in vitro and important for inflammatory pain, as well as for arthritic pain and disease. Here we provide evidence for a nexus between TNF and this pathway, and for TNF and GM-CSF interdependency. We report that the initiation of zymosan-induced inflammatory pain and zymosan-induced arthritic pain and disease are TNF dependent. Once arthritic pain and disease are established, blockade of GM-CSF or CCL17, but not of TNF, is still able to ameliorate them. TNF is required for GM-CSF-driven inflammatory pain and for initiation of GM-CSF-driven arthritic pain and disease, but not once they are established. TNF-driven inflammatory pain and TNF-driven arthritic pain and disease are dependent on GM-CSF and mechanistically require the same downstream pathway involving GM-CSF→CCL17 formation via JMJD3-regulated IRF4 production, indicating that GM-CSF and CCL17 can mediate some of the proinflammatory and algesic actions of TNF. Given we found that TNF appears important only early in arthritic pain and disease progression, targeting a downstream mediator, such as CCL17, which appears to act throughout the course of disease, could be effective at ameliorating chronic inflammatory conditions where TNF is implicated.
Keywords: Arthritis; Cytokines; Inflammation; Macrophages.
Conflict of interest statement
Figures









Similar articles
-
IL-23 in arthritic and inflammatory pain development in mice.Arthritis Res Ther. 2020 May 29;22(1):123. doi: 10.1186/s13075-020-02212-0. Arthritis Res Ther. 2020. PMID: 32471485 Free PMC article.
-
Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation.J Clin Invest. 2016 Sep 1;126(9):3453-66. doi: 10.1172/JCI87828. Epub 2016 Aug 15. J Clin Invest. 2016. PMID: 27525438 Free PMC article.
-
GM-CSF- and IRF4-Dependent Signaling Can Regulate Myeloid Cell Numbers and the Macrophage Phenotype during Inflammation.J Immunol. 2019 May 15;202(10):3033-3040. doi: 10.4049/jimmunol.1801549. Epub 2019 Apr 15. J Immunol. 2019. PMID: 30988114
-
GM-CSF-Dependent Inflammatory Pathways.Front Immunol. 2019 Sep 4;10:2055. doi: 10.3389/fimmu.2019.02055. eCollection 2019. Front Immunol. 2019. PMID: 31552022 Free PMC article. Review.
-
GM-CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential.Expert Rev Clin Immunol. 2015 Apr;11(4):457-65. doi: 10.1586/1744666X.2015.1024110. Epub 2015 Mar 8. Expert Rev Clin Immunol. 2015. PMID: 25748625 Review.
Cited by
-
GM-CSF in inflammation.J Exp Med. 2020 Jan 6;217(1):e20190945. doi: 10.1084/jem.20190945. J Exp Med. 2020. PMID: 31611249 Free PMC article. Review.
-
IL-23 in arthritic and inflammatory pain development in mice.Arthritis Res Ther. 2020 May 29;22(1):123. doi: 10.1186/s13075-020-02212-0. Arthritis Res Ther. 2020. PMID: 32471485 Free PMC article.
-
Randomized, placebo-controlled study on the effects of intravenous GSK3858279 (anti-CCL17) on a battery of evoked pain tests in healthy participants.Clin Transl Sci. 2024 Sep;17(9):e13873. doi: 10.1111/cts.13873. Clin Transl Sci. 2024. PMID: 39250326 Free PMC article. Clinical Trial.
-
Therapeutic blockade of CCL17 in obesity-exacerbated osteoarthritic pain and disease.PLoS One. 2025 Jan 16;20(1):e0317399. doi: 10.1371/journal.pone.0317399. eCollection 2025. PLoS One. 2025. PMID: 39820104 Free PMC article.
-
Effects of swimming training on myocardial protection in rats.Biomed Rep. 2022 Mar;16(3):19. doi: 10.3892/br.2022.1502. Epub 2022 Jan 31. Biomed Rep. 2022. PMID: 35251606 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

