Neuropathic pain in a Fabry disease rat model
- PMID: 29563343
- PMCID: PMC5926911
- DOI: 10.1172/jci.insight.99171
Neuropathic pain in a Fabry disease rat model
Abstract
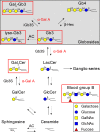
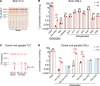
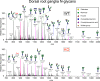
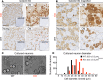
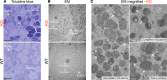
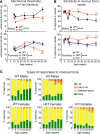
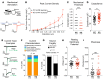
Fabry disease, the most common lysosomal storage disease, affects multiple organs and results in a shortened life span. This disease is caused by a deficiency of the lysosomal enzyme α-galactosidase A, which leads to glycosphingolipid accumulation in many cell types. Neuropathic pain is an early and severely debilitating symptom in patients with Fabry disease, but the cellular and molecular mechanisms that cause the pain are unknown. We generated a rat model of Fabry disease, the first nonmouse model to our knowledge. Fabry rats had substantial serum and tissue accumulation of α-galactosyl glycosphingolipids and had pronounced mechanical pain behavior. Additionally, Fabry rat dorsal root ganglia displayed global N-glycan alterations, sensory neurons were laden with inclusions, and sensory neuron somata exhibited prominent sensitization to mechanical force. We found that the cation channel transient receptor potential ankyrin 1 (TRPA1) is sensitized in Fabry rat sensory neurons and that TRPA1 antagonism reversed the behavioral mechanical sensitization. This study points toward TRPA1 as a potentially novel target to treat the pain experienced by patients with Fabry disease.
Keywords: Genetic diseases; Glycobiology; Neuroscience; Pain.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

