Neural stem cells from a mouse model of Rett syndrome are prone to senescence, show reduced capacity to cope with genotoxic stress, and are impaired in the differentiation process
- PMID: 29563495
- PMCID: PMC6118406
- DOI: 10.1038/s12276-017-0005-x
Neural stem cells from a mouse model of Rett syndrome are prone to senescence, show reduced capacity to cope with genotoxic stress, and are impaired in the differentiation process
Abstract
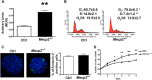
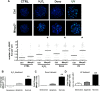
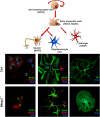
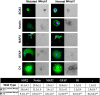
Several aspects of stem cell life are governed by epigenetic variations, such as DNA methylation, histone modifications, and chromatin remodeling. Epigenetic events are also connected with the impairment of stem cell functions. For example, during senescence, there are significant changes in chromatin organization that alter transcription. The MECP2 protein can bind methylated cytosines and contribute to regulating gene expression at one of the highest hierarchical levels. Researchers are particularly interested in this protein, as up to 90% of Rett syndrome patients have an MECP2 gene mutation. Nevertheless, the role of MECP2 in this disease remains poorly understood. We used a mouse model of Rett syndrome to evaluate whether residual MECP2 activity in neural stem cells (NSCs) induced the senescence phenomena that could affect stem cell function. Our study clearly demonstrated that the reduced expression of MECP2 is connected with an increase in senescence, an impairment in proliferation capacity, and an accumulation of unrepaired DNA foci. Mecp2 +/- NSCs did not cope with genotoxic stress in the same way as the control cells did. Indeed, after treatment with different DNA-damaging agents, the NSCs from mice with mutated Mecp2 accumulated more DNA damage foci (γ-H2AX+) and were more prone to cell death than the controls. Senescence in Mecp2 +/- NSCs decreased the number of stem cells and progenitors and gave rise to a high percentage of cells that expressed neither stem/progenitor nor differentiation markers. These cells could be senescent and dysfunctional.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

