AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling
- PMID: 29563504
- PMCID: PMC5862985
- DOI: 10.1038/s41467-018-03582-5
AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling
Abstract
Auxin is a key regulator of plant growth and development, but the causal relationship between hormone transport and root responses remains unresolved. Here we describe auxin uptake, together with early steps in signaling, in Arabidopsis root hairs. Using intracellular microelectrodes we show membrane depolarization, in response to IAA in a concentration- and pH-dependent manner. This depolarization is strongly impaired in aux1 mutants, indicating that AUX1 is the major transporter for auxin uptake in root hairs. Local intracellular auxin application triggers Ca2+ signals that propagate as long-distance waves between root cells and modulate their auxin responses. AUX1-mediated IAA transport, as well as IAA- triggered calcium signals, are blocked by treatment with the SCFTIR1/AFB - inhibitor auxinole. Further, they are strongly reduced in the tir1afb2afb3 and the cngc14 mutant. Our study reveals that the AUX1 transporter, the SCFTIR1/AFB receptor and the CNGC14 Ca2+ channel, mediate fast auxin signaling in roots.
Conflict of interest statement
The authors declare no competing interests.
Figures


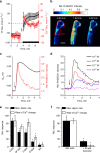

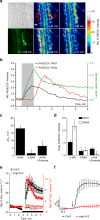
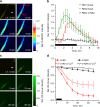
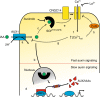
Comment in
-
Low Phosphate Puts Auxin in the Root Hairs.Trends Plant Sci. 2018 Oct;23(10):845-847. doi: 10.1016/j.tplants.2018.07.009. Epub 2018 Aug 7. Trends Plant Sci. 2018. PMID: 30097376
References
-
- Boysen-Jensen P. Über die Leitung des phototropischen Reizes in der Avenakoleoptile. Ber. Deut. Bot. Ges. 1913;31:559–566.
-
- Went FW, White R. Experiments on the transport of auxin. Bot. Gaz. 1939;100:465–484. doi: 10.1086/334804. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

