Applicability of Automated Cell Counter with a Chlorophyll Detector in Routine Management of Microalgae
- PMID: 29563559
- PMCID: PMC5862891
- DOI: 10.1038/s41598-018-23311-8
Applicability of Automated Cell Counter with a Chlorophyll Detector in Routine Management of Microalgae
Abstract
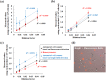
Microalgae have attracted attention for several industrial applications, but all such applications demand culture quality because of their sensitivity to environmental changes. Although simplicity, speed, and accuracy are important to assess algal cultures, researchers have expended vast amounts of labor to monitor algal health using hemocytometry. Along with its user bias, quantifying the cell status aside from the cell density is not easy. This paper describes the easy and rapid evaluation of algal number and status using an image-based cell counter (Countess II FL; Thermo Fisher Scientific Inc.) with a fluorescent filter for chlorophyll. Unlike mammalian cultured cells larger than microalgae, it is not easy for a low-resolution camera alone to distinguish microalgae from grimy spots and microbubbles on counting plates. To assess this method's performance, freshwater/marine microalgae and environmental samples were evaluated using the instrument. Results reveal that an instrument with a fluorescence filter can distinguish microalgae from other particles more precisely than a device with no filter. Values obtained using the instrument were not significantly different from those obtained using hemocytometry. Moreover, the cell counter, but not hemocytometry, can qualify the algal status. Results demonstrate that this system, which has no user bias, can contribute to algal assessment.
Conflict of interest statement
The author declares no competing interests.
Figures







References
-
- Kafarski P. Rainbow code of biotechnology. CHEMIK. 2012;66:811–816.
-
- Arashida R. Characteristics of the microalgae Euglena and its applications in foods and ecological fields. The Jap. Soc. of Photosynthesis Res. 2012;22:33–38.
-
- Hameed MSA, Ebrahim OH. Biotechnological Potential Uses of Immobilized Algae. Int. J. Agric. & Biol. 2007;9:183–192.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

