Impact of prenatal stress on offspring glucocorticoid levels: A phylogenetic meta-analysis across 14 vertebrate species
- PMID: 29563562
- PMCID: PMC5862967
- DOI: 10.1038/s41598-018-23169-w
Impact of prenatal stress on offspring glucocorticoid levels: A phylogenetic meta-analysis across 14 vertebrate species
Abstract
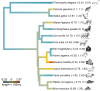
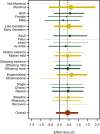
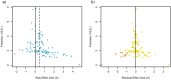
Prenatal exposure to maternal stress is commonly associated with variation in Hypothalamic Pituitary Adrenal (HPA)-axis functioning in offspring. However, the strength or consistency of this response has never been empirically evaluated across vertebrate species. Here we meta-analyzed 114 results from 39 studies across 14 vertebrate species using Bayesian phylogenetic mixed-effects models. We found a positive overall effect of prenatal stress on offspring glucocorticoids (d' = 0.43) though the 95% Highest Posterior Density Interval overlapped with 0 (-0.16-0.95). Meta-regressions of potential moderators highlighted that phylogeny and life history variables predicted relatively little variation in effect size. Experimental studies (d' = 0.64) produced stronger effects than observational ones (d' = -0.01), while prenatal stress affected glucocorticoid recovery following offspring stress exposure more strongly (d' = 0.75) than baseline levels (d' = 0.48) or glucocorticoid peak response (d' = 0.36). These findings are consistent with the argument that HPA-axis sensitivity to prenatal stress is evolutionarily ancient and occurs regardless of a species' overall life history strategy. These effects may therefore be especially important for mediating intra-specific life-history variation. In addition, these findings suggest that animal models of prenatal HPA-axis programming may be appropriate for studying similar effects in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Periconceptional ethanol exposure alters the stress axis in adult female but not male rat offspring.Stress. 2019 May;22(3):347-357. doi: 10.1080/10253890.2018.1563068. Epub 2019 Feb 11. Stress. 2019. PMID: 30741061
-
Maternal high fat diet programs hypothalamic-pituitary-adrenal function in adult rat offspring.Psychoneuroendocrinology. 2019 Apr;102:128-138. doi: 10.1016/j.psyneuen.2018.12.003. Epub 2018 Dec 6. Psychoneuroendocrinology. 2019. PMID: 30544004
-
Prenatal drug exposure and neurodevelopmental programming of glucocorticoid signalling.J Neuroendocrinol. 2020 Jan;32(1):e12786. doi: 10.1111/jne.12786. Epub 2019 Oct 2. J Neuroendocrinol. 2020. PMID: 31469457 Free PMC article. Review.
-
Prenatal maternal psychopathology and stress and offspring HPA axis function at 6 years.Psychoneuroendocrinology. 2019 Jan;99:120-127. doi: 10.1016/j.psyneuen.2018.09.003. Epub 2018 Sep 7. Psychoneuroendocrinology. 2019. PMID: 30223193
-
[Transgenerational effects of prenatal stress of different etiology].Izv Akad Nauk Ser Biol. 2012 Sep-Oct;(5):529-39. Izv Akad Nauk Ser Biol. 2012. PMID: 23136741 Review. Russian.
Cited by
-
Heritable shifts in redox metabolites during mitochondrial quiescence reprogramme progeny metabolism.Nat Metab. 2021 Sep;3(9):1259-1274. doi: 10.1038/s42255-021-00450-3. Epub 2021 Sep 20. Nat Metab. 2021. PMID: 34545253 Free PMC article.
-
COVID-19-related financial stress associated with higher likelihood of depression among pregnant women living in the United States.Am J Hum Biol. 2021 May;33(3):e23508. doi: 10.1002/ajhb.23508. Epub 2020 Sep 22. Am J Hum Biol. 2021. PMID: 32964542 Free PMC article.
-
Chronic stress, epigenetics, and adipose tissue metabolism in the obese state.Nutr Metab (Lond). 2020 Oct 19;17:88. doi: 10.1186/s12986-020-00513-4. eCollection 2020. Nutr Metab (Lond). 2020. PMID: 33088334 Free PMC article. Review.
-
Exposure to Prenatal Stress Is Associated With an Excitatory/Inhibitory Imbalance in Rat Prefrontal Cortex and Amygdala and an Increased Risk for Emotional Dysregulation.Front Cell Dev Biol. 2021 Jun 1;9:653384. doi: 10.3389/fcell.2021.653384. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34141707 Free PMC article.
-
Prenatal programming of environmental sensitivity.Transl Psychiatry. 2023 May 10;13(1):161. doi: 10.1038/s41398-023-02461-y. Transl Psychiatry. 2023. PMID: 37164986 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

