JMJD5 is a human arginyl C-3 hydroxylase
- PMID: 29563586
- PMCID: PMC5862942
- DOI: 10.1038/s41467-018-03410-w
JMJD5 is a human arginyl C-3 hydroxylase
Erratum in
-
Publisher Correction: JMJD5 is a human arginyl C-3 hydroxylase.Nat Commun. 2018 Apr 23;9(1):1675. doi: 10.1038/s41467-018-04196-7. Nat Commun. 2018. PMID: 29686330 Free PMC article.
Abstract
Oxygenase-catalysed post-translational modifications of basic protein residues, including lysyl hydroxylations and Nε-methyl lysyl demethylations, have important cellular roles. Jumonji-C (JmjC) domain-containing protein 5 (JMJD5), which genetic studies reveal is essential in animal development, is reported as a histone Nε-methyl lysine demethylase (KDM). Here we report how extensive screening with peptides based on JMJD5 interacting proteins led to the finding that JMJD5 catalyses stereoselective C-3 hydroxylation of arginine residues in sequences from human regulator of chromosome condensation domain-containing protein 1 (RCCD1) and ribosomal protein S6 (RPS6). High-resolution crystallographic analyses reveal overall fold, active site and substrate binding/product release features supporting the assignment of JMJD5 as an arginine hydroxylase rather than a KDM. The results will be useful in the development of selective oxygenase inhibitors for the treatment of cancer and genetic diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures

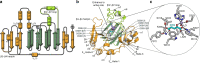


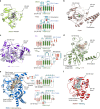

Similar articles
-
The interplay of protein-ligand and water-mediated interactions shape affinity and selectivity in the LAO binding protein.FEBS J. 2020 Feb;287(4):763-782. doi: 10.1111/febs.15019. Epub 2019 Aug 5. FEBS J. 2020. PMID: 31348608
-
Structural analysis of the 2-oxoglutarate binding site of the circadian rhythm linked oxygenase JMJD5.Sci Rep. 2022 Nov 30;12(1):20680. doi: 10.1038/s41598-022-24154-0. Sci Rep. 2022. PMID: 36450832 Free PMC article.
-
Crystal structure and functional analysis of JMJD5 indicate an alternate specificity and function.Mol Cell Biol. 2012 Oct;32(19):4044-52. doi: 10.1128/MCB.00513-12. Epub 2012 Jul 30. Mol Cell Biol. 2012. PMID: 22851697 Free PMC article.
-
Structure of the JmjC-domain-containing protein JMJD5.Acta Crystallogr D Biol Crystallogr. 2013 Oct;69(Pt 10):1911-20. doi: 10.1107/S0907444913016600. Epub 2013 Sep 20. Acta Crystallogr D Biol Crystallogr. 2013. PMID: 24100311
-
Specific Recognition of Arginine Methylated Histone Tails by JMJD5 and JMJD7.Sci Rep. 2018 Feb 19;8(1):3275. doi: 10.1038/s41598-018-21432-8. Sci Rep. 2018. PMID: 29459673 Free PMC article.
Cited by
-
Ribosomal Protein S6: A Potential Therapeutic Target against Cancer?Int J Mol Sci. 2021 Dec 21;23(1):48. doi: 10.3390/ijms23010048. Int J Mol Sci. 2021. PMID: 35008473 Free PMC article. Review.
-
Roles of HIF and 2-Oxoglutarate-Dependent Dioxygenases in Controlling Gene Expression in Hypoxia.Cancers (Basel). 2021 Jan 19;13(2):350. doi: 10.3390/cancers13020350. Cancers (Basel). 2021. PMID: 33477877 Free PMC article. Review.
-
Human 2-oxoglutarate-dependent oxygenases: nutrient sensors, stress responders, and disease mediators.Biochem Soc Trans. 2020 Oct 30;48(5):1843-1858. doi: 10.1042/BST20190333. Biochem Soc Trans. 2020. PMID: 32985654 Free PMC article. Review.
-
Biochemical and structural investigations clarify the substrate selectivity of the 2-oxoglutarate oxygenase JMJD6.J Biol Chem. 2019 Jul 26;294(30):11637-11652. doi: 10.1074/jbc.RA119.008693. Epub 2019 May 30. J Biol Chem. 2019. PMID: 31147442 Free PMC article.
-
A Genetic Map of the Response to DNA Damage in Human Cells.Cell. 2020 Jul 23;182(2):481-496.e21. doi: 10.1016/j.cell.2020.05.040. Epub 2020 Jul 9. Cell. 2020. PMID: 32649862 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

