HIPK2 and extrachromosomal histone H2B are separately recruited by Aurora-B for cytokinesis
- PMID: 29563611
- PMCID: PMC6021368
- DOI: 10.1038/s41388-018-0191-6
HIPK2 and extrachromosomal histone H2B are separately recruited by Aurora-B for cytokinesis
Abstract
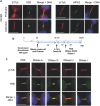
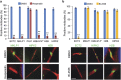
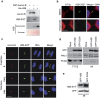
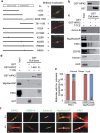
Cytokinesis, the final phase of cell division, is necessary to form two distinct daughter cells with correct distribution of genomic and cytoplasmic materials. Its failure provokes genetically unstable states, such as tetraploidization and polyploidization, which can contribute to tumorigenesis. Aurora-B kinase controls multiple cytokinetic events, from chromosome condensation to abscission when the midbody is severed. We have previously shown that HIPK2, a kinase involved in DNA damage response and development, localizes at the midbody and contributes to abscission by phosphorylating extrachromosomal histone H2B at Ser14. Of relevance, HIPK2-defective cells do not phosphorylate H2B and do not successfully complete cytokinesis leading to accumulation of binucleated cells, chromosomal instability, and increased tumorigenicity. However, how HIPK2 and H2B are recruited to the midbody during cytokinesis is still unknown. Here, we show that regardless of their direct (H2B) and indirect (HIPK2) binding of chromosomal DNA, both H2B and HIPK2 localize at the midbody independently of nucleic acids. Instead, by using mitotic kinase-specific inhibitors in a spatio-temporal regulated manner, we found that Aurora-B kinase activity is required to recruit both HIPK2 and H2B to the midbody. Molecular characterization showed that Aurora-B directly binds and phosphorylates H2B at Ser32 while indirectly recruits HIPK2 through the central spindle components MgcRacGAP and PRC1. Thus, among different cytokinetic functions, Aurora-B separately recruits HIPK2 and H2B to the midbody and these activities contribute to faithful cytokinesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
HIPK2 Phosphorylates the Microtubule-Severing Enzyme Spastin at S268 for Abscission.Cells. 2019 Jul 5;8(7):684. doi: 10.3390/cells8070684. Cells. 2019. PMID: 31284535 Free PMC article.
-
HIPK2 controls cytokinesis and prevents tetraploidization by phosphorylating histone H2B at the midbody.Mol Cell. 2012 Jul 13;47(1):87-98. doi: 10.1016/j.molcel.2012.04.029. Epub 2012 May 31. Mol Cell. 2012. PMID: 22658722
-
CDKL5 localizes at the centrosome and midbody and is required for faithful cell division.Sci Rep. 2017 Jul 24;7(1):6228. doi: 10.1038/s41598-017-05875-z. Sci Rep. 2017. PMID: 28740074 Free PMC article.
-
HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis.Bioessays. 2013 Jan;35(1):55-64. doi: 10.1002/bies.201200060. Epub 2012 Nov 21. Bioessays. 2013. PMID: 23169233 Review.
-
Knowing when to cut and run: mechanisms that control cytokinetic abscission.Trends Cell Biol. 2013 Sep;23(9):433-41. doi: 10.1016/j.tcb.2013.04.006. Epub 2013 May 22. Trends Cell Biol. 2013. PMID: 23706391 Review.
Cited by
-
HIPK2 Is Required for Midbody Remnant Removal Through Autophagy-Mediated Degradation.Front Cell Dev Biol. 2020 Sep 15;8:572094. doi: 10.3389/fcell.2020.572094. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33043004 Free PMC article.
-
Functions of SRPK, CLK and DYRK kinases in stem cells, development, and human developmental disorders.FEBS Lett. 2023 Oct;597(19):2375-2415. doi: 10.1002/1873-3468.14723. Epub 2023 Sep 4. FEBS Lett. 2023. PMID: 37607329 Free PMC article. Review.
-
HIPK2 in the physiology of nervous system and its implications in neurological disorders.Biochim Biophys Acta Mol Cell Res. 2023 Jun;1870(5):119465. doi: 10.1016/j.bbamcr.2023.119465. Epub 2023 Mar 20. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36935052 Free PMC article. Review.
-
HIPK2 Phosphorylates the Microtubule-Severing Enzyme Spastin at S268 for Abscission.Cells. 2019 Jul 5;8(7):684. doi: 10.3390/cells8070684. Cells. 2019. PMID: 31284535 Free PMC article.
-
An Alternative Splice Variant of HIPK2 with Intron Retention Contributes to Cytokinesis.Cells. 2020 Feb 20;9(2):484. doi: 10.3390/cells9020484. Cells. 2020. PMID: 32093146 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

