Inhibition of cell proliferation and migration through nucleobase-modified polyamidoamine-mediated p53 delivery
- PMID: 29563788
- PMCID: PMC5846749
- DOI: 10.2147/IJN.S146917
Inhibition of cell proliferation and migration through nucleobase-modified polyamidoamine-mediated p53 delivery
Abstract
Introduction: The nucleobase 2-amino-6-chloropurine-modified polyamidoamine (AP-PAMAM) was used as a carrier for p53 gene delivery to achieve the antitumor effects.
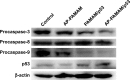
Methods and materials: The condensation of p53 plasmid was studied through gel retardation assay, and the transfection efficiency was evaluated through the transfection assay of pEGFP-N3 and pGL-3 plasmids. Using human cervical carcinoma cell line HeLa as a model, the inhibition of cell proliferation and migration was studied through flow cytometry, wound healing and Transwell migration assays, respectively. The p53 expression level was detected through quantitative polymerase chain reaction and Western blotting analyses.
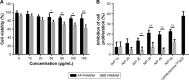
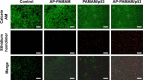
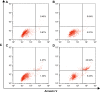
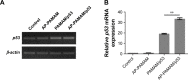
Results: The carrier could condense p53 plasmid into stable nanoparticles at N/P ratios of 2.0, and higher transfection efficiency than polyamidoamine (PAMAM) could be obtained at all the N/P ratios studied. AP-PAMAM-mediated p53 delivery could achieve stronger antiproliferative effect than PAMAM/p53. The antiproliferative effect was identified to be triggered by the induction of cell apoptosis (apoptotic ratio of 26.17%) and cell cycle arrest at S phase. Additionally, AP-PAMAM/p53 transfection has been found to suppress the cell migration and invasion of cancer cells. Finally, the enhanced p53 expression level could be detected after p53 transfection at mRNA and protein levels.
Conclusion: The PAMAM derivative-mediated p53 delivery could be a promising strategy for achieving tumor gene therapy.
Keywords: cell migration; cell proliferation; nucleobase; p53 delivery; polyamidoamine.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






Similar articles
-
A chemoenzymatically synthesized cholesterol-g-poly(amine-co-ester)-mediated p53 gene delivery for achieving antitumor efficacy in prostate cancer.Int J Nanomedicine. 2019 Feb 13;14:1149-1161. doi: 10.2147/IJN.S191905. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30863051 Free PMC article.
-
Nucleobase-modified polyamidoamine-mediated miR-23b delivery to inhibit the proliferation and migration of lung cancer.Biomater Sci. 2017 Oct 24;5(11):2268-2275. doi: 10.1039/c7bm00599g. Biomater Sci. 2017. PMID: 28976503
-
Inhibition of proliferation and migration of tumor cells through phenylboronic acid-functionalized polyamidoamine-mediated delivery of a therapeutic DNAzyme Dz13.Int J Nanomedicine. 2019 Aug 9;14:6371-6385. doi: 10.2147/IJN.S211744. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31496692 Free PMC article.
-
Co-delivery of p53 and MDM2 inhibitor RG7388 using a hydroxyl terminal PAMAM dendrimer derivative for synergistic cancer therapy.Acta Biomater. 2019 Dec;100:118-131. doi: 10.1016/j.actbio.2019.09.041. Epub 2019 Sep 27. Acta Biomater. 2019. PMID: 31568878
-
Phenylboronic Acid-Modified Polyamidoamine Mediated the Transfection of Polo-Like Kinase-1 siRNA to Achieve an Anti-Tumor Efficacy.Int J Nanomedicine. 2021 Dec 13;16:8037-8048. doi: 10.2147/IJN.S329433. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34934312 Free PMC article.
Cited by
-
Salinomycin and Sulforaphane Exerted Synergistic Antiproliferative and Proapoptotic Effects on Colorectal Cancer Cells by Inhibiting the PI3K/Akt Signaling Pathway in vitro and in vivo.Onco Targets Ther. 2020 Jun 3;13:4957-4969. doi: 10.2147/OTT.S246706. eCollection 2020. Onco Targets Ther. 2020. PMID: 32581555 Free PMC article.
-
Melittin alcalase-hydrolysate: a novel chemically characterized multifunctional bioagent; antibacterial, anti-biofilm and anticancer.Front Microbiol. 2024 Jul 17;15:1419917. doi: 10.3389/fmicb.2024.1419917. eCollection 2024. Front Microbiol. 2024. PMID: 39091304 Free PMC article.
-
Targeted Gene Delivery Therapies for Cervical Cancer.Cancers (Basel). 2020 May 21;12(5):1301. doi: 10.3390/cancers12051301. Cancers (Basel). 2020. PMID: 32455616 Free PMC article. Review.
-
A chemoenzymatically synthesized cholesterol-g-poly(amine-co-ester)-mediated p53 gene delivery for achieving antitumor efficacy in prostate cancer.Int J Nanomedicine. 2019 Feb 13;14:1149-1161. doi: 10.2147/IJN.S191905. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30863051 Free PMC article.
-
Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy.Int J Mol Sci. 2021 Mar 13;22(6):2912. doi: 10.3390/ijms22062912. Int J Mol Sci. 2021. PMID: 33805602 Free PMC article. Review.
References
-
- Bakhtiar A, Sayyad M, Rosli R, Maruyama A, Chowdhury EH. Intracellular delivery of potential therapeutic genes: prospects in cancer gene therapy. Curr Gene Ther. 2014;14(4):247–257. - PubMed
-
- Joerger AC, Fersht AR. The p53 pathway: origins, inactivation in cancer, and emerging therapeutic approaches. Annu Rev Biochem. 2016;85:375–404. - PubMed
-
- Mantovani F, Walerych D, Sal GD. Targeting mutant p53 in cancer: a long road to precision therapy. FEBS J. 2017;284(6):837–850. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

