Design of pH-sensitive methotrexate prodrug-targeted curcumin nanoparticles for efficient dual-drug delivery and combination cancer therapy
- PMID: 29563794
- PMCID: PMC5849920
- DOI: 10.2147/IJN.S152312
Design of pH-sensitive methotrexate prodrug-targeted curcumin nanoparticles for efficient dual-drug delivery and combination cancer therapy
Abstract
Aim: We designed acid-labile methotrexate (MTX) targeting prodrug self-assembling nanoparticles loaded with curcumin (CUR) drug for simultaneous delivery of multi-chemotherapeutic drugs and combination cancer therapy.
Methods: A dual-acting MTX, acting as both an anticancer drug and as a tumor-targeting ligand, was coupled to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[aldehyde(polyethylene glycol)-2000] via Schiff's base reaction. The synthesized prodrug conjugate (DSPE-PEG-Imine-MTX) could be self-assembled into micellar nanoparticles (MTX-Imine-M) in aqueous solution, which encapsulated CUR into their core by hydrophobic interactions (MTX-Imine-M-CUR).
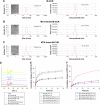
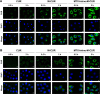
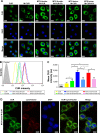
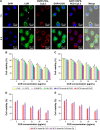
Results: The prepared MTX-Imine-M-CUR nanoparticles were composed of an inner hydrophobic DSPE/CUR core and an outside hydrophilic bishydroxyl poly (ethyleneglycol) (PEG) shell with a self-targeting MTX prodrug corona. The imine linker between 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[aldehyde(polyethyleneglycol)-2000] and MTX, as a dynamic covalent bond, was strong enough to remain intact in physiological pH, even though it is rapidly cleaved in acidic pH. The MTX-Imine-M-CUR could codeliver MTX and CUR selectively and efficiently into the cancer cells via folate receptor-mediated endocytosis followed by the rapid intracellular release of CUR and the active form of MTX via the acidity of endosomes/lysosomes. Moreover, the MTX-Imine-M-CUR resulted in significantly higher in vitro and in vivo anticancer activity than pH-insensitive DSPE-PEGAmide-MTX assembling nanoparticles loaded with CUR (MTX-Amide-M-CUR), MTX unconjugated DSPE-PEG assembling micellar nanoparticles loaded with CUR (M-CUR), combination of both free drugs, and individual free drugs.
Conclusion: The smart system provided a simple, yet feasible, drug delivery strategy for targeted combination chemotherapy.
Keywords: combination therapy; nanoparticles; pH-sensitive prodrug; self-assembly; targeting.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Lachelt U, Wittmann V, Muller K, et al. Synthetic polyglutamylation of dual-functional mtx ligands for enhanced combined cytotoxicity of poly(I:C) nanoplexes. Mol Pharmaceutics. 2014;11:2631–2639. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–651. - PubMed
-
- Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347:159–166. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

