Efficacy, tolerability, and safety of oral paliperidone extended release in the treatment of schizophrenia: a 24-week, open-label, prospective switch study in different settings in Taiwan
- PMID: 29563800
- PMCID: PMC5848663
- DOI: 10.2147/NDT.S161186
Efficacy, tolerability, and safety of oral paliperidone extended release in the treatment of schizophrenia: a 24-week, open-label, prospective switch study in different settings in Taiwan
Abstract
Purpose: Paliperidone extended release (ER) is an oral psychotropic treatment formulated to release paliperidone at a controlled, gradually ascending rate. We evaluated the efficacy and safety of switching to paliperidone ER in Taiwanese patients with schizophrenia who were unresponsive or intolerant to previous antipsychotic therapy.
Patients and methods: This was a 24-week, open-label, single-arm, multicenter, Phase IV trial. Based on consulting psychiatrists' judgment, patients were deemed eligible for the switch to paliperidone ER; the switch was achieved by cross-tapering, using a recommended starting dose of 6 mg. Eligibility considerations included lack of efficacy, tolerability, and/or adherence to previous oral antipsychotic medication.
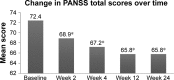
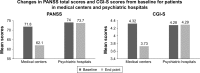
Results: Of the 297 enrolled patients, 178 (59.5%) completed the study. The main reasons for discontinuation included insufficient efficacy (8.7%), patient decision (8.4%), and adverse events (AEs; 6.4%). Improvements in the: Positive and Negative Syndrome Scale total score and Clinical Global Impression-Severity score were observed only in patients treated at medical centers and not in those treated at psychiatric hospitals. The most common AEs were insomnia, headache, constipation, and extrapyramidal syndrome. One or more serious AEs were reported in 11 (3.7%) patients; none resulted in death. No significant changes in body weight, plasma glucose, or lipid levels were observed.
Conclusion: Switching to paliperidone ER was effective and well tolerated for up to 24 weeks in patients with schizophrenia who were unresponsive or intolerant to previous antipsychotic therapy. The observed differences in treatment between psychiatric hospitals and medical centers with regard to dosage and titration of paliperidone ER warrant further investigation.
Keywords: open-label; paliperidone extended release; prospective; schizophrenia; settings; switch study.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Switching from oral risperidone to flexibly dosed oral paliperidone extended-release: core symptoms, satisfaction, and quality of life in patients with stable but symptomatic schizophrenia: the RISPALI study.Curr Med Res Opin. 2014 Apr;30(4):695-709. doi: 10.1185/03007995.2013.869201. Epub 2013 Dec 16. Curr Med Res Opin. 2014. PMID: 24289141 Clinical Trial.
-
Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension.Am J Geriatr Psychiatry. 2008 Jan;16(1):31-43. doi: 10.1097/JGP.0b013e31815a3e7a. Am J Geriatr Psychiatry. 2008. PMID: 18165460 Clinical Trial.
-
Paliperidone extended release: a review of its use in the management of schizophrenia.Drugs. 2010 Jul 9;70(10):1295-317. doi: 10.2165/11204840-000000000-00000. Drugs. 2010. PMID: 20568835 Review.
-
Efficacy and safety of paliperidone extended release in adolescents with schizophrenia: a randomized, double-blind study.J Am Acad Child Adolesc Psychiatry. 2015 Feb;54(2):126-137.e1. doi: 10.1016/j.jaac.2014.11.009. Epub 2014 Nov 25. J Am Acad Child Adolesc Psychiatry. 2015. PMID: 25617253 Clinical Trial.
-
Extended-release paliperidone: efficacy, safety and tolerability profile of a new atypical antipsychotic.Drugs Today (Barc). 2007 Apr;43(4):249-58. doi: 10.1358/dot.2007.43.4.1067342. Drugs Today (Barc). 2007. PMID: 17460786 Review.
Cited by
-
Second-Generation Antipsychotic Drugs for Patients with Schizophrenia: Systematic Literature Review and Meta-analysis of Metabolic and Cardiovascular Side Effects.Clin Drug Investig. 2021 Apr;41(4):303-319. doi: 10.1007/s40261-021-01000-1. Epub 2021 Mar 9. Clin Drug Investig. 2021. PMID: 33686614 Free PMC article.
References
-
- Valenstein M, Ganoczy D, McCarthy JF, Myra Kim H, Lee TA, Blow FC. Antipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective review. J Clin Psychiatry. 2006;67:1542–1550. - PubMed
-
- Yamada K, Watanabe K, Nemoto N, et al. Prediction of medication noncompliance in outpatients with schizophrenia: 2-year follow-up study. Psychiatry Res. 2006;141:61–69. - PubMed
-
- Cooper C, Bebbington P, King M, et al. Why people do not take their psychotropic drugs as prescribed: results of the 2000 National Psychiatric Morbidity Survey. Acta Psychiatr Scand. 2007;116:47–53. - PubMed
-
- Nasrallah HA, Targum SD, Tandon R, McCombs JS, Ross R. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56:273–282. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

