Proportion of medication error reporting and associated factors among nurses: a cross sectional study
- PMID: 29563855
- PMCID: PMC5848571
- DOI: 10.1186/s12912-018-0280-4
Proportion of medication error reporting and associated factors among nurses: a cross sectional study
Abstract
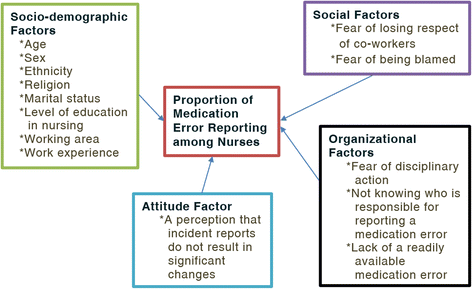
Background: A medication error (ME) is any preventable event that may cause or lead to inappropriate medication use or patient harm. Voluntary reporting has a principal role in appreciating the extent and impact of medication errors. Thus, exploration of the proportion of medication error reporting and associated factors among nurses is important to inform service providers and program implementers so as to improve the quality of the healthcare services.
Methods: Institution based quantitative cross-sectional study was conducted among 397 nurses from March 6 to May 10, 2015. Stratified sampling followed by simple random sampling technique was used to select the study participants. The data were collected using structured self-administered questionnaire which was adopted from studies conducted in Australia and Jordan. A pilot study was carried out to validate the questionnaire before data collection for this study. Bivariate and multivariate logistic regression models were fitted to identify factors associated with the proportion of medication error reporting among nurses. An adjusted odds ratio with 95% confidence interval was computed to determine the level of significance.
Result: The proportion of medication error reporting among nurses was found to be 57.4%. Regression analysis showed that sex, marital status, having made a medication error and medication error experience were significantly associated with medication error reporting.
Conclusion: The proportion of medication error reporting among nurses in this study was found to be higher than other studies.
Keywords: Federal Level Governmental Hospital; Medication error; Medication error reporting; Nurse.
Conflict of interest statement
Ethical clearance and approval was obtained from Ethical Review Committee of School of Nursing, College of Medicine and Health Sciences, University of Gondar and permission was obtained from the Ethical Review Committees of each respective hospital. Consent form was put as a first page of each questionnaire, and included the name of the researcher, the purpose of the study, and a number of ethically based instructions. Participants were assured that their involvement in the study was after having been informed about the study without undue influence and could withdraw from the study at any time without the need to give reason. The privacy of the participants was maintained while they fill the questionnaire and confidentiality of the participants was maintained by keeping anonymity and keeping the data only accessible by the investigator. Although there may not be immediate and direct benefits for the participants, nurses were informed of the benefits from the nursing knowledge gained through the process. The participants were informed that there were no financial benefits for participating in the research, no potential harms that impact on employment, or social status, the utilization of the gathered data to be used only for the intended research, and the publication of the results of the study in a reputable journal with no identifiable information that links to the participants.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources