Soluble Fms-Like Tyrosine Kinase-1 Alters Cellular Metabolism and Mitochondrial Bioenergetics in Preeclampsia
- PMID: 29563877
- PMCID: PMC5845757
- DOI: 10.3389/fphys.2018.00083
Soluble Fms-Like Tyrosine Kinase-1 Alters Cellular Metabolism and Mitochondrial Bioenergetics in Preeclampsia
Abstract
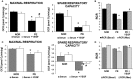
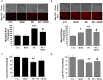
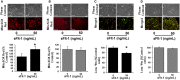
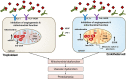
Preeclampsia is a maternal hypertensive disorder that affects up to 1 out of 12 pregnancies worldwide. It is characterized by proteinuria, endothelial dysfunction, and elevated levels of the soluble form of the vascular endothelial growth factor receptor-1 (VEGFR-1, known as sFlt-1). sFlt-1 effects are mediated in part by decreasing VEGF signaling. The direct effects of sFlt-1 on cellular metabolism and bioenergetics in preeclampsia, have not been established. The goal of this study was to evaluate whether sFlt-1 causes mitochondrial dysfunction leading to disruption of normal functioning in endothelial and placental cells in preeclampsia. Endothelial cells (ECs) and first-trimester trophoblast (HTR-8/SVneo) were treated with serum from preeclamptic women rich in sFlt-1 or with the recombinant protein. sFlt-1, dose-dependently inhibited ECs respiration and acidification rates indicating a metabolic phenotype switch enhancing glycolytic flux. HTR-8/SVneo displayed a strong basal glycolytic metabolism, remaining less sensitive to sFlt-1-induced mitochondrial impairment. Moreover, results obtained in ECs exposed to serum from preeclamptic subjects demonstrated that increased sFlt-1 leads to metabolic perturbations accountable for mitochondrial dysfunction observed in preeclampsia. sFlt-1 exacerbated mitochondrial reactive oxygen species (ROS) formation and mitochondrial membrane potential dissipation in ECs and trophoblasts exposed to serum from preeclamptic women. Forcing oxidative metabolism by culturing cells in galactose media, further sensitized cells to sFlt-1. This approach let us establish that sFlt-1 targets mitochondrial function in ECs. Effects of sFlt-1 on HTR-8/SVneo cells metabolism were amplified in galactose, demonstrating that sFlt-1 only target cells that rely mainly on oxidative metabolism. Together, our results establish the early metabolic perturbations induced by sFlt-1 and the resulting endothelial and mitochondrial dysfunction in preeclampsia.
Keywords: endothelial dysfunction; metabolism; mitochondrial dysfunction; oxidative stress and metabolic perturbations; preeclampsia; sFlt-1.
Figures



References
-
- American College Obstetricians Gynecologist and Task Force on Hypertension in Pregnancy (2013). Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 122, 1122–1131. 10.1097/01.AOG.0000437382.03963.88 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

