Linking Associations of Rare Low-Abundance Species to Their Environments by Association Networks
- PMID: 29563898
- PMCID: PMC5850922
- DOI: 10.3389/fmicb.2018.00297
Linking Associations of Rare Low-Abundance Species to Their Environments by Association Networks
Abstract
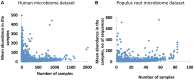
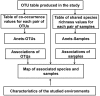
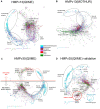
Studies of microbial communities by targeted sequencing of rRNA genes lead to recovering numerous rare low-abundance taxa with unknown biological roles. We propose to study associations of such rare organisms with their environments by a computational framework based on transformation of the data into qualitative variables. Namely, we analyze the sparse table of putative species or OTUs (operational taxonomic units) and samples generated in such studies, also known as an OTU table, by collecting statistics on co-occurrences of the species and on shared species richness across samples. Based on the statistics we built two association networks, of the rare putative species and of the samples respectively, using a known computational technique, Association networks (Anets) developed for analysis of qualitative data. Clusters of samples and clusters of OTUs are then integrated and combined with metadata of the study to produce a map of associated putative species in their environments. We tested and validated the framework on two types of microbiomes, of human body sites and that of the Populus tree root systems. We show that in both studies the associations of OTUs can separate samples according to environmental or physiological characteristics of the studied systems.
Keywords: Anets; alpha and beta diversity; metagenome; microbiome; qualitative data; sparse data; unsupervised analysis.
Figures





References
-
- Ashelford K. E., Chuzhanova N. A., Fry J. C., Jones A. J., Weightman A. J. (2005). At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl. Environ. Microbiol. 71 7724–7736. 10.1128/aem.71.12.7724-7736.2005 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

