Chronic Inflammatory Microenvironment in Epidermodysplasia Verruciformis Skin Lesions: Role of the Synergism Between HPV8 E2 and C/EBPβ to Induce Pro-Inflammatory S100A8/A9 Proteins
- PMID: 29563902
- PMCID: PMC5845987
- DOI: 10.3389/fmicb.2018.00392
Chronic Inflammatory Microenvironment in Epidermodysplasia Verruciformis Skin Lesions: Role of the Synergism Between HPV8 E2 and C/EBPβ to Induce Pro-Inflammatory S100A8/A9 Proteins
Abstract
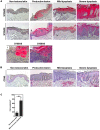
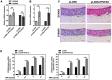
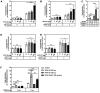
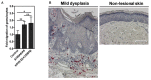
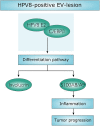
Persistent genus β-HPV (human papillomavirus) infection is a major co-factor for non-melanoma skin cancer in patients suffering from the inherited skin disease epidermodysplasia verruciformis (EV). Malignant EV lesions are particularly associated with HPV type 5 or 8. There is clinical and molecular evidence that HPV8 actively suppresses epithelial immunosurveillance by interfering with the recruitment of Langerhans cells, which may favor viral persistence. Mechanisms how persistent HPV8 infection promotes the carcinogenic process are, however, less well understood. In various tumor types chronic inflammation has a central role in tumor progression. The calprotectin complex consisting of S100A8 and S100A9 proteins has recently been identified as key driver of chronic and tumor promoting inflammation in skin carcinogenesis. It induces chemotaxis of neutrophil granulocytes and modulates inflammatory as well as immune responses. In this study, we demonstrate that skin lesions of EV-patients are massively infiltrated by inflammatory cells, including CD15+ granulocytes. At the same time we observed a very strong expression of S100A8 and S100A9 proteins in lesional keratinocytes, which was mostly confined to the suprabasal layers of the epidermis. Both proteins were hardly detected in non-lesional skin. Further experiments revealed that the HPV8 oncoproteins E6 and E7 were not involved in S100A8/A9 up-regulation. They rather suppressed differentiation-induced S100A8/A9 expression. In contrast, the viral transcription factor E2 strongly enhanced PMA-mediated S100A8/A9 up-regulation in primary human keratinocytes. Similarly, a tremendous up-regulation of both S100 proteins was observed, when minute amounts of the PMA-inducible CCAAT/enhancer binding protein β (C/EBPβ), which is expressed at low levels in the suprabasal layers of the epidermis, were co-expressed together with HPV8 E2. This confirmed our previous observation that C/EBPβ interacts and functionally synergizes with the HPV8 E2 protein in differentiation-dependent gene expression. Potent synergistic up-regulation of S100A8/A9 was seen at transcriptional and protein levels. S100A8/A9 containing supernatants from keratinocytes co-expressing HPV8 E2 and C/EBPβ significantly induced chemotaxis of granulocytes in migration assays supporting the relevance of our finding. In conclusion, our data suggest that the HPV8 E2 protein actively contributes to the recruitment of myeloid cells into EV skin lesions, which may support chronic inflammation and progression to skin cancer.
Keywords: C/EBP; E2; HPV; S100A8/A9; epidermodysplasia verruciformis; inflammation.
Figures

References
-
- Akgul B., Karle P., Adam M., Fuchs P. G., Pfister H. J. (2003). Dual role of tumor suppressor p53 in regulation of DNA replication and oncogene E6-promoter activity of epidermodysplasia verruciformis-associated human papillomavirus type 8. Virology 308 279–290. 10.1016/S0042-6822(02)00133-2 - DOI - PubMed
-
- Balasubramanian S., Agarwal C., Efimova T., Dubyak G. R., Banks E., Welter J., et al. (2000). Thapsigargin suppresses phorbol ester-dependent human involucrin promoter activity by suppressing CCAAT-enhancer-binding protein alpha (C/EBPalpha) DNA binding. Biochem. J. 350(Pt 3) 791–796. 10.1042/bj3500791 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

